Last week a massive spill of red sludge from an aluminum production company in Hungary killed several people and destroyed a tremendous amount of property. Here’s a look at what this red sludge is and why it’s so bad.

Bauxite ore
The sludge is a byproduct of a process used to extract aluminaa precursor of aluminum metal from a type of ore called bauxite. Bauxite contains several things besides alumina, including silicon dioxiderelated to sand and glass, iron oxides'rust', and titanium dioxide. To separate alumina from these other components, the bauxite is crushed up and added to a boiling hot solution of sodium hydroxideChemical formula: NaOH. Sometimes called 'lye' and water. Sodium hydroxide is a chemical that is a strong base; this means that when it is dissolved in water, the solution is extremely alkaline and caustic (you would not want to put your hand in this). The sodium hydroxide reacts with the alumina to make a more soluble form of aluminum (aluminum hydroxide), which dissolves into the water. The rest of the material – the silicon, iron, and titanium oxides – doesn’t dissolve and just sits there as sludge.
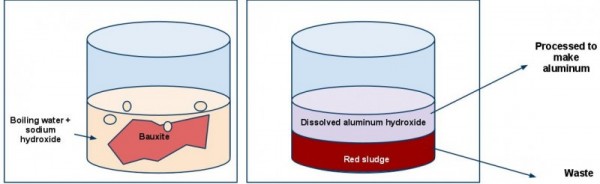
This sludge has a reddish color – probably largely due to all the iron oxides (remember, that is essentially what rust is). The solution above the sludge contains the aluminum, and this solution is removed and further processed to make aluminum metal. The sludge, on the other hand, is left behind and has to be disposed of.
As you might imagine, this sludge isn’t very pleasant. Because it was produced in a solution of sodium hydroxide in water, it is extremely alkaline itself. Contact with skin would result in severe chemical burns. If you put a little bit of this sludge in a fishtank, it would raise the pH of the tank (make it more alkaline); as everyone who has ever owned a fishtank knows – this means death for the fish.

