The chemistry Nobel Prize was announced this morning, and my colleagues and I are thrilled that it went to some real organometallic chemists! Even better, they were honored for their use of my favorite metal, palladium.

Akira Suzuki, Ei-ichi Negishi, and Richard Heck are sharing the 2010 Nobel prize for “palladium-catalyzed cross couplings in organic synthesis”. This phrase sounds like a mouthful, but it’s really not so bad when you break it down.
In loose terms, the three of them independently invented similar, extremely useful reactions to create new carbon-carbon bonds. The skeletons of all organic moleculesnearly all molecules with any relevancy to living things are made of carbon atoms, so it’s crucially important for organic chemists to have simple, reliable, and versatile methods to make carbon-carbon bonds in the lab. The term “cross-coupling” simply means joining (coupling) together carbon atoms of two different molecules (as opposed to two identical molecules). The Suzuki reaction, the Negishi reaction, and the Heck reaction have some subtle differences that make them complementary to each other, but in general all three reactions look like the following:
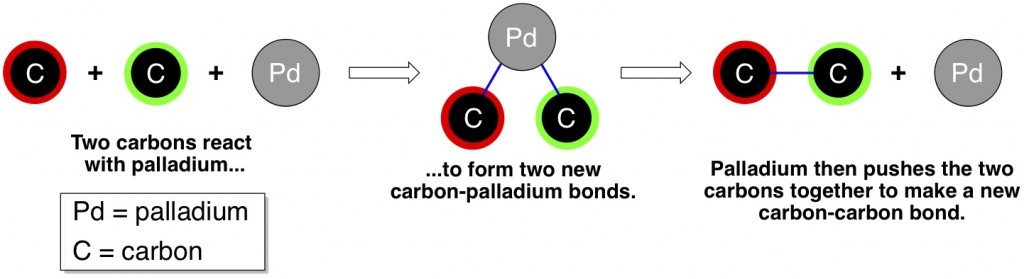
The palladium plays a key role – the two carbons in the above scheme don’t bond to each other without some hand-holding by palladium. Of course, the reactions are a bit more complicated than this cartoon depicts. For example, not just any carbon will react with palladium – it needs to have certain other atoms already bonded to it. Regardless, these coupling reactions have been enormously useful to make all kinds of molecules, like pharmaceuticals, agrochemicals, natural products, and the everyday sorts of molecules that graduate students have to make in lab.

