 Like acetone in a previous post, acetic acid is another common laboratory chemical that makes frequent cameo appearances in your home. Here are 10 – dare I say – “fun” facts about acetic acid that you may or may not know.
Like acetone in a previous post, acetic acid is another common laboratory chemical that makes frequent cameo appearances in your home. Here are 10 – dare I say – “fun” facts about acetic acid that you may or may not know.
1. Acetic acid’s claim to fame at home is vinegar. Vinegar is typically a 4-8% solution of acetic acid; the rest is water and trace amounts of other molecules that impart slightly different flavors to different types of vinegar (balsamic is my favorite).
2. While the name acetic acid is accepted by IUPACInternational Union of Pure and Appplied Chemistry; they make up the chemistry nomenclature rules, you can also call it ethanoic acid if you strictly follow the basic IUPAC naming system. Doesn’t have as nice a ring to it though.
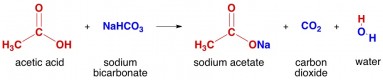
 3. One of the most important uses for household acetic acid is to make a volcano science project. All you need is a volcano model, some vinegar, some baking soda (sodium bicarbonate), and probably some red food coloring if you really want the molten lava look. The acetic acid reacts with the sodium bicarbonate to generate a bunch of CO2 – this bubbles off to give the volcano effect.
3. One of the most important uses for household acetic acid is to make a volcano science project. All you need is a volcano model, some vinegar, some baking soda (sodium bicarbonate), and probably some red food coloring if you really want the molten lava look. The acetic acid reacts with the sodium bicarbonate to generate a bunch of CO2 – this bubbles off to give the volcano effect.
4. The acetic acid in vinegar is formed by the fermentation of ethanol. This reaction requires oxygen from the air, which is why opened bottles of wine taste vinegary after a while, even though unopened bottles of wine can keep for ages.
5. There is an estimated global demand for acetic acid of 6.5 million metric tons. This comes out to about 1 kilo of acetic acid per person. If this was due to vinegar consumption, this value would correspond to 5 gallons of vinegar per person per year. That is clearly not accurate; it turns out most of the acetic acid in the world is being used for other things…
6. The main use for acetic acid is to make vinyl acetate, which can then be used to make the polymer polyvinylacetate, or PVA. This polymer is used in many glues, including yellow “carpenter’s glue”.
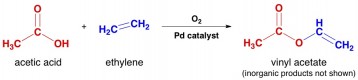
7. Most acetic acid is not made by fermentation. Industrially, it is usually made from methanol by the Monsanto process, which features a rhodium catalyst for a key carbonylative step.
 8. Concentrated acetic acid is often called “glacial acetic acid”. The origin of this name can be attributed to acetic acid’s high freezing point. Acetic acid freezes into a solid at a temperature very close to room temperature: 16.5 ºC, or 62 ºF. When it freezes, it kind of looks like ice; hence the term “glacial”.
8. Concentrated acetic acid is often called “glacial acetic acid”. The origin of this name can be attributed to acetic acid’s high freezing point. Acetic acid freezes into a solid at a temperature very close to room temperature: 16.5 ºC, or 62 ºF. When it freezes, it kind of looks like ice; hence the term “glacial”.
9. At home, vinegar has some very practical uses besides salad dressing. It can be used to remove limescale – the ugly white minerals that accumulate over time from hard water. For example, tea kettles and shower heads can benefit from a vinegar soak.
 10. One of the most effective ways to wash fruits and veggies is with dilute vinegar. In one study, a dilute vinegar wash removed 98% of the bacteria from fruit skins, compared to 85% removal with water and a scrub brush alone.
10. One of the most effective ways to wash fruits and veggies is with dilute vinegar. In one study, a dilute vinegar wash removed 98% of the bacteria from fruit skins, compared to 85% removal with water and a scrub brush alone.


love the science project facts!!:)
i need to know what is better conductor of an electricity a base or a acid
I had no idea washing fruit with vineagar is one of the most effective ways! That’s some great info. Love hearing fun chemistry and chemical facts.