 I first discovered the ethanol puppy when I was a sophomore in college. While exploring the cupboards of a dingy office that I shared with the other lab TAs, I found this dusty old box with yellowed papers dating from the 1970s. At the bottom of the box were a few pieces of an old wooden space-filling molecule kit. Unlike traditional ball-and-stick molecule kits (where ball-shaped atoms are connected by sticks that represent bonds), a space filling model smooshes all the atoms up next to each other. It’s a little more accurate representation of the three-dimensional shape of a molecule.
I first discovered the ethanol puppy when I was a sophomore in college. While exploring the cupboards of a dingy office that I shared with the other lab TAs, I found this dusty old box with yellowed papers dating from the 1970s. At the bottom of the box were a few pieces of an old wooden space-filling molecule kit. Unlike traditional ball-and-stick molecule kits (where ball-shaped atoms are connected by sticks that represent bonds), a space filling model smooshes all the atoms up next to each other. It’s a little more accurate representation of the three-dimensional shape of a molecule.
As in most model kits, the atoms were color coded – there were black carbon atoms, hideous orange hydrogens, and baby blue oxygen atoms. The wooden pieces snap together:

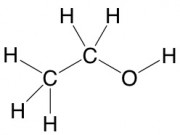
There were enough pieces to make a simple organic molecule: C2H6O, or ethanol (as in, alcohol). This is what ethanol looks like when you draw it the normal organic chemistry way (kind of boring):
This is what ethanol looks like when you use a space-filling model kit:
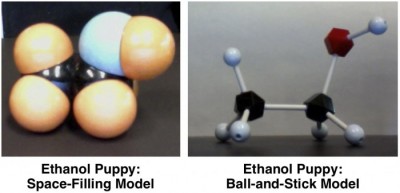
A puppy!

