Hypothetically, let’s say that (1) I graduate someday and (2) my parents come out for my thesis defense. Since I am, in fact, not curing cancer, I fear that my defense will be a snoozer for any non-chemists such as said parents. Thus, I’ve decided to begin preparing them for the occasion. (I have not yet informed them of this.) In addition to abundant coffee and perhaps a “Where’s Waldo” hidden in my PowerPoint slides, it seems beneficial to provide them with a glossary of terms they might hear.![]()
I’ll begin with a few of them here, and more will likely follow in the future at some point. The ones in this post are all related to methods of analyzing chemicals to figure out what they are. They’re all acronyms that are technically nouns but are often used informally as verbs.* Since I’m an organic chemist, this is an organic chemist’s perspective on chemical analysis. A true analytical chemist would probably feel the need to say a lot more on these topics.
GC
Purpose: A GC is a device that helps you figure out how many chemicals are present in a mixture, and can allow you to determine how much is present of each chemical.

How it Works: The user injects a small amount of sample into the GC with a syringe. The injected sample is instantly heated up really hote.g., 250 ºC, or 482 ºF such that everything vaporizes into a gas. The volatilized chemicals must then travel through a really longe.g., 15 m, super-narrow coiled tube called a GC column. The inside of the column is a little sticky to molecules, and the overall effect is that some types of chemicals take longer to get through the column and some take a shorter time. When any molecules finally exit the column, a detector takes note of their passing and records the time. The end result is a graph (called a chromatogram), that might look something like this:
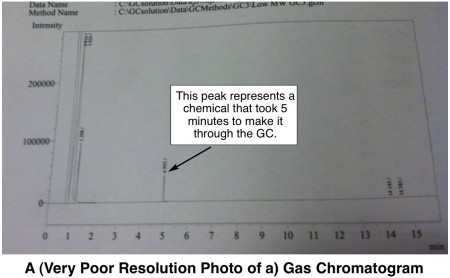
The area under each peak is proportional to the amount of chemical present in the original sample. Thus, a GC can not only tell you how many different chemicals are present in a sample, it can tell you how much there is of each.
A graph from a GC won’t necessarily give you any information about the identity of your chemicals though. But, if you have an idea of what should be in your sample, you can compare the chromatogram of your sample to chromatograms of known chemicals and see if they match up with respect to how many minutes it takes to travel through the GC.
GC-MS
Purpose: A GC-MS is a device that gives you the same information as a GC (above), but also helps you to figure out the identity of chemicals.
How it Works. In addition to a regular GC component, a GC-MS has a special fancy detector that tells you the mass of each molecule that comes through. Thus it gives you some extra information that you can’t get from a regular GC. If you know the mass of a molecule, that narrows down the possibilities for its identity by a lot! For example, water’s mass is ~18 (because oxygen weighs 16 amuatomic mass units and hydrogen weighs 1 amuatomic mass units). If something in your chromatogram has a mass of 56, you can bet it’s not water.
NMR
Purpose: An NMR is a device that gives you a lot of information about the structure of a chemical. In many cases you can conclusively identify the exact ordering of all the atoms in a molecule. It is also an important technique for analyzing the purity of a sample.
How it Works. An NMR is the chemist’s version of an MRI (like at the doctor’s office). It’s a pretty big device that contains a very powerful magnet. Any molecules that are placed inside the magnetic field will actually respond to the magnet on a sub-atomic level – even molecules that you wouldn’t normally think of as “magnetic”, such as water.

![]()
To analyze a chemical by NMR, you dissolve a small amount of it in a special solvent and put the solution into a hollow glass tube. The tube is inserted into the NMR, and then the user sits down at a computer and tells the computer to run the instrument.
There are many different ways to analyze a molecule by NMR. The simplest procedure is to just look at how hydrogens (more appropriately called protons) interact with the magnetic field, ignoring all other types of atoms. Alternatively, you can choose to look at carbon, fluorine, phosphorus, or many other atoms. The end result of “taking an NMR” is a graph – called a spectrum – that looks something like this:
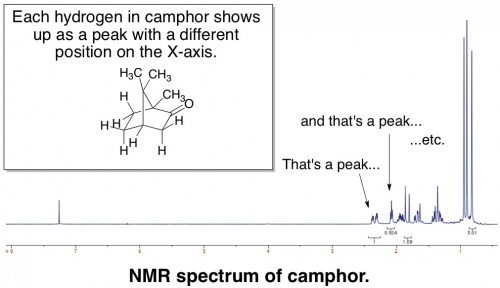
Each peak represents a hydrogen or a group of hydrogens, and its placement on the X-axis reflects its relative positioning in a molecule. NMR is probably the most powerful analytical tool that organic chemists use, and you absolutely must analyze every organic chemical you make by NMR before you can publish anything about it.
*Disclaimer:
The use of any of these words as verbs is for slang purposes only. It happens all the time in the lab, but woe to the careless graduate student that blurts out “I NMRed my sample” during his candidacy exam. He can expect his committee members to correct him: “No, you did not NMR your sample, you analyzed your sample by NMR spectroscopy.”


…or that could have different connotations if you’re like my husband. To his ears, “I have to go get an NMR,” always turns into “I have to go get an enema.”
While it sometimes feels like I’ve had an enema after meeting with my PI, these are, in fact, two very different things.
Speaking for moms everywhere, I thank you for this clear explanation. My daughter the lawyer gives me similar explanations of obscure legal terms.