Someday in the distant future, I hope to be able to explain my own research project to my parents without making up lies about how I’m curing cancer. Perhaps if they understand my chemistry a bit, they won’t feel the need to bring a novel to read during my thesis defenseshould they be able to come, of course, no pressure : ), assuming I ever get to that point.
So here’s a start – a discussion about mirrors.
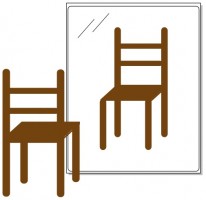
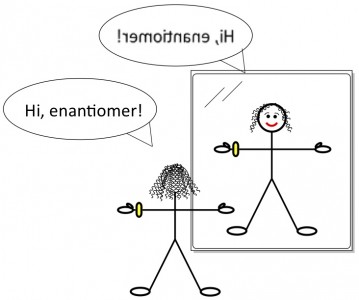
You’re standing in front of a mirror (Figure 1) looking at your reflection, perhaps admiring the look of the new gold Rolex that graces your wrist. (OK, in my family maybe it’s a new GPS watch or something). Then, like Carroll’s Alice, you step into the mirror. You turn around to face out, now standing shoulder to shoulder with your mirror twin (Figure 2). But something is amiss. The watch that you wear on your left wrist is instead worn on your twin’s right. You part your hair on the right, but your twin parts hers on her left. The two of you aren’t identical, and try as you might to twist and rotate your body, you can’t make yourself match up with your reflection.
A person who knows you well can discern between you and your reflection, perhaps by recalling the placement of a dimple or a freckle. Some objects, however, are completely indistinguishable from their reflections. Take the example of a simple, perfectby perfect I mean symmetrical, not that this is the ideal look for household furnishing kitchen chair:
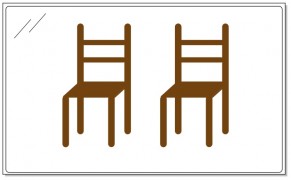
You can perform the same exercise with this chair – push it through the mirror and turn it around to face front. As you stand back and scrutinize the two chairs that stand side by side, you will not be able to tell the difference between the two. The original chair and its reflection are completely superimposable.
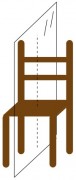
The difference between you and the chair is one of symmetry. The chair is perfectly symmetrical from one side to the other – the left side is exactly like the right. In fact, the left side is the mirror image of the right side. You can imagine a vertical plane slicing right through the center of the chair, such that everything on the right side of the plane is the mirror image of everything on the left.
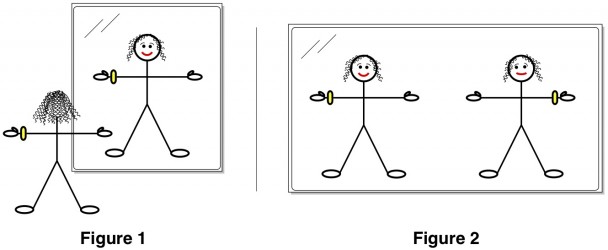
This is not the case for you (“you” being the stick figure above). Your left side is not the mirror image of your right, because your left wrist has a watch on it and your right does not. Of course, in reality, you have many slight imperfections that make even your unaccessorized body asymmetricalAnd in truth, a real life chair would also have such imperfections..
The concept that some things are identical to their mirror images and others are not is a fundamental principle in organic chemistry. The adjective “chiral” (ch is pronounced like k, and the word rhymes with eye-roll) is used to describe molecules that are not identical twins with their mirror images.
A chiral molecule can’t be superimposed on its mirror image, no matter how you rotate it. You could say that your body is chiral.
An achiral molecule cannot be distinguished from its mirror image. The chair in the picture above is achiral.
There’s another key vocabulary word related to mirrors and chemistry. The noun enantiomer is a slightly shorter way of saying “non-superimposable mirror image.” Your body and your reflection are enantiomers of each other. All chiral molecules have enantiomers, which are unique molecules in their own right. An achiral molecule doesn’t have an enantiomer; its mirror image has the exact same identity as itself.
Why do chemists care about mirror images? It turns out that nearly every molecule in biological systems (e.g., in your body) is chiral. Often only one enantiomer – either a molecule or its mirror image – works properly to carry out a given biological function.
This is important to consider when medicinal chemists design pharmaceutical drugs. For example, the active ingredient in the sleep aid Lunesta is a chiral molecule. Though this molecule helps people with insomnia, the mirror image version of this molecule doesn’t do a darn thing. In other cases, the mirror image of a medicine’s active ingredient is actually harmful. Therefore, even before getting to the drug-design stage, a lot of fundamental research (cough cough mine) in chemistry labs deals with developing new methods to selectively make one chiral molecule without making much of its enantiomer.
Whew. This is not going to be easyI didn't even touch on diastereomers, tetrahedrons, chiral centers...…


As I’ve mentioned before the story of thalidomide is not as straightforward as it is often made out to be…
Thanks Stu, that’s a good point! For those of you who haven’t heard – the “bad” enantiomer of thalidomide causes birth defects, but unfortunately the “good” enantiomer can/does convert into the bad one upon ingestion. (Jargon term: it racemizes.) Click the link above for detail!!
I guess I should have made it clear that the blogpost linked to in my comment above was not written by me, but I left a comment that the author of the blogpost kindly worked into the original post…
hehehe … I liked the eye-roll gag!
: )
Friend sent me here today…cute post. I may use this example for teaching! If you really care about being able to make complex ideas simple, there is a place in the world for you! I have made my career out of being a good technical chemist with a knack for talking to young students and lay people…and it’s a lot of fun!
Thanks for reading and for the encouragement!
I get it! I get it! And so YOUR job is to figure out how to miniaturize Rolex watches (OK, GPS watches) so they’ll fit on your wee little molecules, right? How cute would that be? Those GPS watches would help them find each other more easily in the vastness of intermolecular space, too.
Hmmm. Close enough. : )
Ah, enantiomers. The bane of any undergrad organic student.
Having read the other post you wrote on the “here’s what I do” topic, I’m curious – are you working on methodology or toward a specific synthetic molecule goal?
Just curious…
Ha! And the bane of many a graduate student!
It’s methodology : )
Awww. I’m an undergrad in Pharmaceutical Chemistry and I think Chiral centers are fascinatingly interesting.
I guess it all depends on how well you are able to envision 3D structures in you head.
@Daemon – You’re right, 3D mental rotation ability is key – video games actually might help out here! Chiral centers ARE quite interesting. HOWever… they can be just a teensy little bit frustrating when you’re trying to make them with high stereochemical purity in lab! ; )
Excellent explanation of enantiomers! It really is a talent to be able to describe complex topics to a lay audience. Now, how to boil it down to a 10-second “elevator speech”? 🙂 As an environmental scientist, I’ve always had somewhat weird jobs, and describing them to people outside the industry is a challenge.
Fantastic! I may send some of my o chem students here. They have this problem of understanding enantiomers and really why the hell why organic chemistry is important, and why its useful. You really address that well here. Great post!
Thanks! It would make me happy if it’s useful to someone : )
One of the few things I remember from organic chemistry was a comment that an enantiomer of sucrose (or fructose) might have the same flavor but not have the same number of calories. Anyone know if this is an active area of research?
i’m a major skincare fanatic and that relates to this post because there are a couple skincare lines out there who tout their products as being best for your skin because they are chiral. i had a slight understanding of it just from googling and reading a few different things, but this example you give is just so clear and brilliant! if anyone didn’t completely ‘get’ chirality i would send them right here! if i, a woefully undereducated layperson could get it from your article, anyone should, and that means something to be able to communicate to the average person such a complex tidbit!