 What would happen, hypothetically, if a baby mouse could grow up eating only deuterated food and water? Could you make an unusually heavy mouse?
What would happen, hypothetically, if a baby mouse could grow up eating only deuterated food and water? Could you make an unusually heavy mouse?
Isotopes
The concept of a deuterated mouse requires a little explanation about isotopes. (Skip this section if you already know what an isotope is.)
The identity of any particular element is decided by the number of protons (positively charged particles) that the atom contains in its nucleus. For example, all carbon atoms – by definition – contain 6 protons. All oxygen atoms contain 8 protons, and all hydrogen atoms contain just one proton.
However, some carbon atoms are heavier than other carbon atoms. The same is true for hydrogen, oxygen, and all the other elements as well. This is because the mass of an atom takes into account not only protons, but another type of nuclear particle called a neutron (which weighs about the same as a proton). Most carbon atoms contain 6 neutrons in addition to their 6 protons, but some carbon atoms contain 7 or even 8 neutrons.
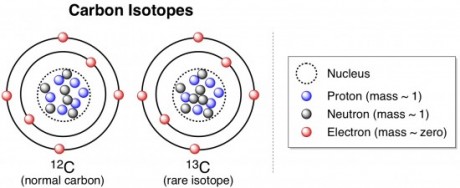
Thus, you can say that carbon exists as three different isotopes. Same element, different mass. There’s 12C (pronounced “carbon-twelve”), which is the most abundant carbon isotope in the universe, and has a mass of 12units = atomic mass units because of its 6 protons and 6 neutrons. Then there’s the more rare 13C, which has 6 protons and 7 neutrons, and the really rare and radioactive 14C, with 6 protons and 8 neutrons.
Deuterium
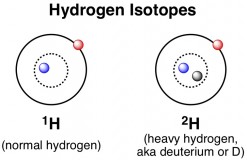
Like carbon, hydrogen exists as more than one isotope. Most hydrogen atoms contain zero neutrons in addition to their one proton, but about 1 in every 6400 hydrogen atoms contains a single neutron as well. This heavy isotope of hydrogen can be called 2H, but it is commonly referred to by the name “deuterium” or “D”. Whereas most hydrogen atoms have a mass of 1, deuterium weighs (nearly) exactly double, with a mass of 2. Thus, although water made with deuterium (D2O) looks and tastes just like regular water, it is called “heavy water” because each molecule weighs substantially more.
A Heavy Mouse
Like any life form, mice contain a lot of hydrogen atoms. Besides all the H2O in and around the mouse cells, nearly every other molecule in a mouse’s body includes hydrogen atoms.
So what would happen if a mouse ate only deuterated food and water? Instead of drinking H2O, the mouse drank only D2O. Instead of eating normal proteins, the mouse ate only protein made from amino acids whose hydrogens had been replaced with deuteriums.
 The baby mouse would grow up to be a heavy mouse, right? Not a fat mouse, just a more dense mouse. About 10% of a mouse’s weight is due to hydrogen atomsat least, this is the figure Wikipedia cites for a human body. Thus, replacing all the hydrogen atoms in a mouse’s body with deuteriums would result in a 10% weight increase, since deuterium weighs twice as much as hydrogen.
The baby mouse would grow up to be a heavy mouse, right? Not a fat mouse, just a more dense mouse. About 10% of a mouse’s weight is due to hydrogen atomsat least, this is the figure Wikipedia cites for a human body. Thus, replacing all the hydrogen atoms in a mouse’s body with deuteriums would result in a 10% weight increase, since deuterium weighs twice as much as hydrogen.
So, a 20 gram mouse would weigh 22 grams if he were deuterated.
A couple extra grams might not sound like a lot, but it’s equivalent to a 150 pound person putting on 15 pounds, without having to go up a clothing size!
The Problem with Deuterating a Mouse
To cut to the punchline, a mouse (or any mammal) couldn’t live off of only deuterated food/water. Cellular functions don’t work when all, or even half, of the body’s hydrogens have been replaced with deuterium.
But why not? Deuterium is still hydrogen. It’s not a unique element – it doesn’t get its own position on the periodic table. It’s not radioactive. Any bond that hydrogen can make, so can deuterium.
It turns out that chemical bonds involving deuterium atoms are a bit slower to break than bonds involving hydrogens.
 And bond breaking is very important for life. The bonds between atoms in food and water molecules have to get ripped apart so that a mouse body can reassemble the atoms as muscle tissue or brain cells. Every single metabolic pathway you can remember having to memorize in biology class (Krebs cycleaka citric acid cycle, on the right) requires numerous bond breaking events.
And bond breaking is very important for life. The bonds between atoms in food and water molecules have to get ripped apart so that a mouse body can reassemble the atoms as muscle tissue or brain cells. Every single metabolic pathway you can remember having to memorize in biology class (Krebs cycleaka citric acid cycle, on the right) requires numerous bond breaking events.
The timing of all these chemical reactions has to be just so for everything to work right. Thus, if the rate of bond-breaking is slowed down significantly, cells won’t be able to function properly and will start to die.
Kinetic Isotope Effect
The reason that bonds involving deuterium break slower than those involving hydrogen has to do with a concept called a kinetic isotope effect (KIE). This concept is an absolute favoritesecond only to, perhaps, Hammett plots of physical organic chemists.
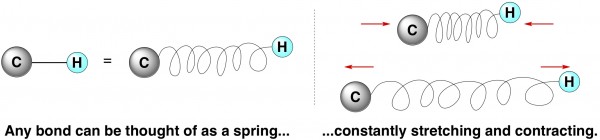
The idea of a KIE goes something like this. A bond between any two atoms can be thought of as a spring, with an atom attached to both ends. This spring is constantly stretching and contracting, in and out, over and over. Some bonds stretch and contract very quickly and frequently, like a really bouncy spring. Others are slower, with fewer stretches happening over a given period of time.
If you think about it, the first step of breaking a bond (or a flimsy plastic spring) is to stretch it. Thus, a bouncy bond that has a high frequency of stretching is going to be inherently faster to break than a bond that naturally stretches infrequently.
The frequency of stretching of any given bond can be calculated mathematically by using the classic physics equation for stretching a spring with a mass attached to both ends. I’m going to skip overcan't say I understand it completely anyway that math, but suffice to say that the weight of the two end pieces is an important term in the equation. The stretching frequency is inversely proportional to masstechnically to the square root of the reduced mass, so stretching becomes slower as the mass at either end becomes heavier.
Thus, since deuterium is twice as heavy as hydrogen, a bond involving deuterium has a lower stretching frequency than one involving hydrogen. This makes it slower to break.*
It turns out that the difference in bond-breaking rate can be pretty serious just from this tiny mass difference. For example, a carbon–deuterium bond can be 6.5 times slower to break than a carbon-hydrogen bond.** No wonder deuterium throws off the timing of cellular chemical reactions!
But There Is Still (Meager) Hope for a Heavy Mouse
OK, so replacing all the hydrogens in a mouse’s body with the twice-as-heavy deuterium won’t work because of the kinetic isotope effect. But maybe we could still play with isotopes of a different atom.
All the carbon in a mouse body, which is mostly 12C, could theoretically be replaced with 13C. Whereas deuterium weighs 100% more than hydrogen, 13C only weighs about 8% more than the naturally abundant 12C. Thus, maybe this relatively small difference in mass wouldn’t be enough to slow cellular bond-breaking processes down to the point of failure.
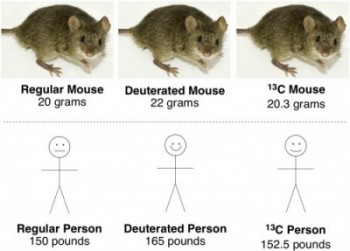
Downside number one, of course, is that a 13C-labeled mouse wouldn’t be as noticeably heavier than a deuterated mouse. Carbon makes up about 18% of the mass of a mammal’s body. If 13C weighs 8.3% more than regular 12C, then a 13C-labeled mouse would only weigh about 1.5% more than a regular mouse – a 20 gram mouse would weigh 20.3 grams. This is like a 150 pound person gaining two and a half pounds. Which isn’t nearly as impressive as 15 pounds.
Downside number two is that a 13C-labeled mouse would be obnoxiously expensive. 13C-labeled glucose (sugar) costs $114.40 per half a gram if you buy it from Cambridge Isotopes. I’m pretty sure a mouse can eat like 3 grams of fooddo mice like to eat sugar? a day. At that price, the mouse might as well be eating three meals a day at the French Laundry for its entire life.


So reactions happen 6.5x slower. I can see this being a problem if percent deuterium incorporation increased to 100% immediately. But I wonder what would happen if one slowly increased the deuterium concentration over time (several generations time). Perhaps the animal would adapt and just take life more slowly – this would be a problem for predator-evasion purposes, but not necessarily for living purposes. Or perhaps the intracellular concentration of proteins would increase to compensate for the decrease in reaction time.
C’mon biologists… get to work! And let me know what happens in 10-15 years!
I’m pretty sure the problem is that life-sustaining reactions such as cellular metabolism are too slow on the molecular level for the mouse to live with only deuterium. Life requires chemistry that happens lightning-fast even on chemical timescales.
I don’t think the body is this sensitive though. For example the ranges of temperature we live in probably changes the speeds of every reaction in the body by more than +-10%. Considering the extra work the mouse will be doing, it would be exerting more energy anyway and be warmer, or it could just be kept in a warmer environment to counter balance the change in reaction speeds. I think it is possible to have a deuterated mouse.
This was an interesting read, but I’m troubled by some of David’s ideas about homeostasis.
Long story short, internal reactions don’t occur faster due to being placed in a hotter environment. You’ll notice your metabolism doesn’t increase when you’re in 100 degree weather as opposed to 90 degree weather. This is because your body keeps itself in a narrow range of temperatures which are required to keep your body’s metabolic rates at normal levels. When the organism is pushed outside of these ranges, they either suffer from hypothermia or hyperthermia, and if not corrected, permanent damage and death can result.
Awesome article though. 5/5, would read again.
Ben’s right:
Average body temp is 37 C. 10% is 3.7 C. So even if you are bouncing between hypothermia (35 C) and a fever (40 C), you’re temperature isn’t changing that much.
Source:
http://www.theaveragebody.com/average_body_temperature.php
I’ve always wondered why pure heavy water would not support life. I wonder if it would quench thirst if a person was stranded on a planet which only had heavy water to drink.
This has been done in rats with 15N. I’m not sure how much it affected their weight on a larger scale, but it the weight increase was noticeable enough for detection/differentiation via mass spectrometry. The rats were fine, but they definitely were eating some super expensive meals (15N enriched algae).
http://pubs.acs.org/doi/abs/10.1021/ac049208j
My comment / question above was answered in a Wikipedia article about Deuterium. So please disregard.
But thinking about the increased mass of a hydrogen atom with a neutron added and just considering the approximate doubling of its mass, it is easier for me to understand why it would be slower with regard to its involvement in (metabolic) chemical reactions, if I make an analogy comparing a hydrogen atom which becomes a deuterium atom, to a person (as done with the heavy water-watered mouse) by comparing the (parenthetical) latter to a 150 lb person who gained 15 lbs:
If a normal hydrogen atom were compared to a tiny-framed person who normally weighs a petite 97 lbs when dripping wet stepping out of a non heavy water shower, then the mass of a deuterium atom would be comparable to that same formerly petite person who had gained an additional 97 lbs and weighed 194 lbs before waddling into or after stepping out of a shower.
At 194 lbs that formerly petite, and still tiny-framed person would no longer be able to participate in something such as hiking club activities and might need a wheelchair to navigate the aisles of a grocery store.
In other words, that person would move much more slowly and would require much more energy to walk the same distance as when being of normal, petite weight.
This made me lol.
Part of it’s lol-ability might be credited to it’s awkwardly flawed composition which was due to constraints of writing it on a cellphone with distractions flurrying about.
But speaking of lolling, I wonder if there’s been research on chemistry of laughter.
Since laughter necessitates a body and bodies are organizations of organic organs, the associated chemistry should be highly organicistic in a supercalifragilistic degree.
David Jones (of Daedalus and DREADCO fame in Nature/New Scientist column Ariadne) has some interesting thoughts about how a deuterated organism would interact with alcohol in Chemistry World a year or so ago http://www.rsc.org/chemistryworld/restricted/2009/October/LastRetort.asp. The full article requires RSC membership, but here’s a taster…
Alcohol makes us drunk. It is a slow-acting anaesthetic, sabotaging the brain, releasing our lower nature, and tempting us to easy emotionalism. It upsets our balance, too – one theory is that alcohol reduces the density of bodily fluids. The semicircular canals of the ear, which govern our balance, therefore lose their balancing skill. Deuterium oxide (heavy water, D2O) also upsets our balance. It acts in just the opposite way to alcohol, increasing the density of our fluids. Hence it should be possible to devise a ‘heavy gin’ with just the right amount of deuterium oxide in it to keep the endolymph fluid in the semicircular canals at the proper density. A drinker would get as emotional as ever, but his balance would not be upset.
@azmanam – I like this hypothesis about mice adapting over many generations, though I wonder if it would take a lot longer than 10-15 years. I feel bad for the poor grad student who gets stuck with that research project.
Turns out that some prokaryotes (algae & bacteria) have been shown capable of growing in heavy water, incorporating deuterium into their biomolecules:
http://www.nrcresearchpress.com/doi/abs/10.1139/y99-005
@Sarah – Even if the 15N-labeled mouse doesn’t feel noticeably heavier when one holds it, this is an excellent proof of principle!!
@David – I really appreciate your optimistic attitude toward being able to get a deuterated mouse, but I’m afraid I agree with Ben’s assessment. Our bodies (and mice’s) are remarkably adept at maintaining homeostasis. Like chemgirl said, the chemical rxns in our cells happen lightning fast, and 6.5 times slower than lightning is no longer lightning.
@Phillip – That is pretty interesting; I’d love to see the breakdown in numbers. A heavy gin might not require all that much heavy water, since ultimately only a very small fraction of alcohol gets into your blood/inner ear fluids. Or if you don’t like gin, you could use heavy club soda as a mixer instead!
Isn’t a chemical reaction at least partially dependent on the mass of the particles at work? The mouse probably wouldn’t be heavier so much as eat less food to get the same amount of energy.
Relevant article:
Mice
http://www.springerlink.com/content/q23x142n72472447/
Dogs:
http://ajplegacy.physiology.org/content/201/2/357.abstract
http://www.sciencedirect.com/science/article/pii/001448276290201X
“The experimental dog was a purebred, one-year-old beagle maintained on 50 per cent deuterium oxide in the drinking water for six and one-half days, and on 75 per cent deuterium oxide for five and one-half days plus additional deuterium oxide in the food [4]. The dog died on the 13th day when the level of deuterium in the body fluids ranged from 33 to 35 per cent.”
I have to agree with what Ben said.
I also must agree that it is an interesting read, just not sure if the science supports it totally.
Could this experiment actually be carried out…? Apart from the cost issue with the isotope. Would the baby mammal just die of dehydration after a few days?
I have been working with deuterium sulphate(Cellfood) for about 10 years, with health with amazing results. A missed point is that when a neutron is added to hydrogen, the neutron (a proton and electron combined) creates a tetrahedral positioning of the components of the atom. This unbalanced atom has double the spin rate (double the energy) of Hydrogen. This speeds up the reaction rate of bonds within living organisms. Deuterium also “splits water” causing both hydrogen ions to dissociate from the oxygen giving both oxidation and reduction at the same time. The cleansing and repairing releases vast amounts of toxins which is extremely tiring to the organism. A balance needs to be made not to take much D2O so that more energy is gain and the detoxification is slowed down. The deuterium will make the food we eat, medicines, the food supplements we take, all work better in the body.
We have been demonstrating that persons getting healthier with a multi-dimensional approach get both bigger and stronger bodies.
About the dog’s death when fed D2O, apart from the possibility of a toxic over load, the massive release of electrolytes alters electrical conductivity, so a supply high quality ions is necessary to balance this. Then the level of D2O used would have altered the fluid pH considerably. Another factor is that a deuterium nuclei has very different magnetic properties to Hydrogen. With body fluids raged from 33 to 35 per cent D2O then this might have also affected the ability of the haemoglobin to function properly.
Ken, excellent information. Is it possible that we humans have caused the lowered deuterium ratio on planet E? All life, carbon based, not just humans.
If that is possible, then the signs of life on another planet would be a reduced deuterium to hydrogen ratio, something like E even tho humans have only been part of the picture since about 5 my ago. And our local gas cloud Fluff is 10 my old according to NASA. Is there a connection?
“… but it the weight increase was noticeable enough for detection/differentiation via mass spectrometry. ”
I can’t help but chuckle at the idea of mass spectrometry of rats – fire them out of a cannon at a wall and see at what height they splat? 🙂
I think Ken needs to read some science books….