 Take a look at the ingredient list on the back of a Sour Patch Kids bag the next time you go to the movie theater, and you probably won’t be surprised to discover that this gummy candy contains a lot of chemicals. With such a complex ingredient list, you might be surprised to learn that the key feature of these snacks – their sourness – is actually quite simple. The sourness comes not from one chemical, not even one element, but from just one tiny little fraction of an atom.
Take a look at the ingredient list on the back of a Sour Patch Kids bag the next time you go to the movie theater, and you probably won’t be surprised to discover that this gummy candy contains a lot of chemicals. With such a complex ingredient list, you might be surprised to learn that the key feature of these snacks – their sourness – is actually quite simple. The sourness comes not from one chemical, not even one element, but from just one tiny little fraction of an atom.
Yup, protons are sour.
Protons are fractions of hydrogen atoms. When a hydrogen atom loses its electron, all that remains is a naked proton, often designated H+ because protons are positively charged.
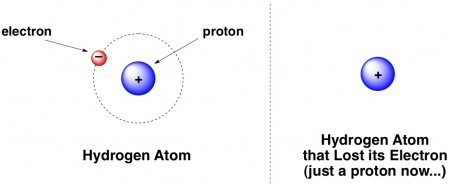
Obligatory chemistry joke that most people have probably heard:
A hydrogen atom walks into a bar. He says to the bartender, “I think I lost my electron.” The bartender asks, “are you sure?” The hydrogen says “I’m positive.”
Moving on.
The amount of naked hydrogen atoms – protons – running about in a mixture (such as in your candy-filled mouth) is directly related to the concept of acidity. By definition, when an acid is placed in a watery solution (such as in saliva), it increases the concentration of protons in that solution, usually because it contains a proton that can “fall off” into the water.*
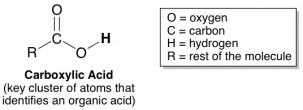
Our tongues detect high concentrations of protons as being sour. Thus, acids taste sour. There are tons of different types of acids out there, but fortunately, most acids that show up in food products are organic acids. Which means they’re fairly easy to recognize if you know their chemical structure. Organic acids are characterized by a cluster of atoms arranged just like this:
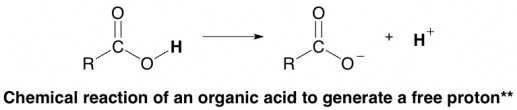
The hydrogen atom in that cluster is prone to falling off, a chemical reaction that can be written like this:**
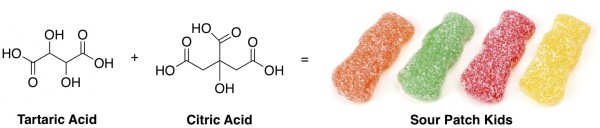
The acids in Sour Patch Kids that donate protons to your tongue are tartaric acid and citric acid, which look like this:
Another important acid for sour foods is lactic acid. This is the acid that is formed in the pickling process, and is responsible for the sour taste of sauerkraut and – of course – pickles.

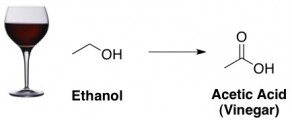
Finally, when wine goes sour it’s because alcohol (ethanol) has turned into vinegar (acetic acid):


Brilliant piece about sour sweets! Have just finished 3 weeks of holiday activities for families all about the science of sweets including “sour” especially citric acid. Managed to get hold of some Nerds which I came across when I taught in the US but hadn’t seen over here in UK. Also used popping candy to take the parents back to their childhood. It worked wonderfully in my show with an adult as a volunteer using a microphone for us all to enjoy the fun in their mouth! So glad to see someone else thinks a little like me!
Great article on food chemistry. Other food references in a funny way in my HAZCOM Song on youtube
http://bit.ly/e88T9g
If one were to aim an electron gun at the surface of a glass of slightly sour wine and stir the wine while zapping it with a stream of electrons…anybody have an opinion on whether that could neutralize the sourness of that glass of wine? (it would, of course, depend on how long it was zapped and what setting was used on the phaser- I mean, the electron gun).
@ Sue Halliday
Also used popping candy to take the parents back to their childhood.
Wow this idea really took me back to my childhood and reminded me of how much fun I and my friends would have eating those little things. Really is fascinating to learn where all the sourness really comes from. Dont suppose all those artificial things are really good for you are they?
Honestly speaking, I really suck when it comes to Chemistry, lol.