As part of my endeavor to figure out how to explain what I do to non-chemists without resorting to lies and vague generalities, I sat down and thought about atoms and molecules. Though it was a big conceptual jump when I first began studying chemistry in high school, once I “got” the idea of atoms and molecules, everything else seemed like it was going to be more manageable.
So how much explanation does it take for someone to sort of “get” the idea of atoms and molecules? Well here goes, an attempt to boil down everything you need to know about atoms and molecules to “get it”, in 500 words or less. This explanation won’t earn you an A on any chemistry exam, but it’s just enough knowledge to be dangerousnot really, please don’t start a meth lab. And by the way, hover your mouse over any blue wordslike these to access notes.
Atoms. Everyone’s heard of atoms – those tiny little particles that make up everything. There are over 100 different types of atoms out there, which seems like a lot if you have to memorize them, but it’s very little if you think about the infinite number of thingspeople, cars, cookies... the list goes on, obviously that these atoms can make. Even more mind boggling is that a lot of what you encounter on a day-to-day basis is made almost entirely from just four of these types of atoms – carbon, hydrogen, oxygen, and nitrogen.
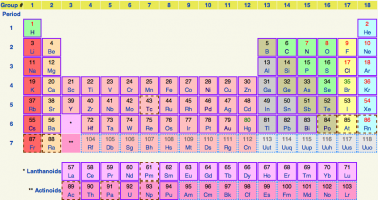
The periodic table graphically represents all the different types of atoms.
Individual atoms of most elementsdifferent 'types' of atoms are called elements aren’t content to be alonethe explanation for why they don’t want to be alone ultimately turns out to be complicated math that nobody really understands perfectly, so they “bond” to other atoms. To think about how bonding works, you have to know just a bit more about atoms.
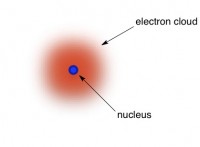 Every atom has a tiny little core, called a nucleus, that is fundamentally different from one element to the next. Surrounding the tiny nucleus is a giant cloud made of electronsthese are sort of particles, sort of just bits of energy; as the name implies, they are related to electricity. Each type of atom has a certain number of electrons associated with it, but the electrons flit about so unimaginably fast that they’re practically everywhere at once (like a cloud) – at least, within a certain radius from the inner core. The electrons are what do the “bonding”.
Every atom has a tiny little core, called a nucleus, that is fundamentally different from one element to the next. Surrounding the tiny nucleus is a giant cloud made of electronsthese are sort of particles, sort of just bits of energy; as the name implies, they are related to electricity. Each type of atom has a certain number of electrons associated with it, but the electrons flit about so unimaginably fast that they’re practically everywhere at once (like a cloud) – at least, within a certain radius from the inner core. The electrons are what do the “bonding”.
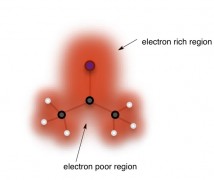 Molecules. When atoms like carbon, hydrogen, oxygen, and nitrogen form bonds, their electron clouds squish together and combine to make one big cloud. You can think of any molecule as having a continuous cloud of electrons that envelopes all of the atoms. The cloud is pretty blotchy though. Some parts are really densethe electrons spend most of their time around there with electrons, and others are quite thinthe electrons spend very little of their time in that region. Note that molecular structures aren’t usually drawn with cloud shapes; instead a single bond is represented by a simple line connecting two letters that represent atoms (bottom of page).
Molecules. When atoms like carbon, hydrogen, oxygen, and nitrogen form bonds, their electron clouds squish together and combine to make one big cloud. You can think of any molecule as having a continuous cloud of electrons that envelopes all of the atoms. The cloud is pretty blotchy though. Some parts are really densethe electrons spend most of their time around there with electrons, and others are quite thinthe electrons spend very little of their time in that region. Note that molecular structures aren’t usually drawn with cloud shapes; instead a single bond is represented by a simple line connecting two letters that represent atoms (bottom of page).
Chemical Reactions. Any time two atoms form or break a bond, it’s called a chemical reaction. This happens when one part of a molecule’s electron cloud that is particularly electron-dense spies a part of another electron cloud that is particularly thin. Electron-rich cloud regions are attracted to electron-poor regions, and if the conditions are right, this attraction will lead to a new bond being formed (and maybe an old one being broken).
Organic chemistry. Generally, organic chemistry is the term used for the study of molecules that feature carbon. Carbon is important because it forms the skeletons of almost all molecules related to living systems. The important thing to know about carbon, as well as the other popular atomshydrogen, nitrogen, and oxygen in organic chemistry, is that each type of atom prefers to form a certain number of bonds with other atoms. Carbon makes four bonds, nitrogen makes three, oxygen makes two, and hydrogen makes just one. If you draw an organic molecule with atoms that don’t follow those rules, chances are that the molecule is strictly imaginary or else it’s highly unstable. Here is a moleculecaffeine where all the atoms are content. Oh, and predictably, C = carbon, H = hydrogen, N = nitrogen, and O = oxygen.
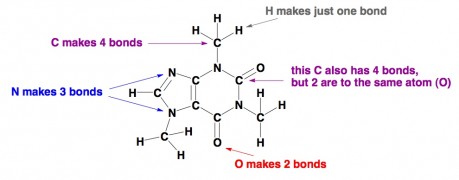


NITRO GROUPS…. 5 BONDS!
Disagree. The correct way to draw a nitro group is with 4 bonds to N. The N has a formal + charge and one of the O’s will have a formal – charge. There are two resonance structures that you can draw that look like this, switching which O has the – charge (and single bond to N), and which O has the neutral charge (and double bond to N).