Winter is finally commencing its retreat in my part of the country, which means we’re actually getting a little sunshine! Clear blue skies usually inspire thoughts of barbecues or pool parties, but they could just as relevantly inspire thoughts about chemistry. (Especially because it’s still too cold for much outdoor funand since I am a graduate student who has no time for barbecues and pool parties.)
Sunlight is one of those rare things that is NOT a chemical. Nevertheless, it is actually the instigator of many chemical reactions. Different wavelengths of light (corresponding to different colors in the visible spectrum) have different amounts of energy associated with them. You can think of each color of light as being capable of delivering a different sized packet of energy to a molecule. Many molecules are responsive to such energy packets and, if exposed to just the right wavelength(s) of light, will undergo a chemical change.
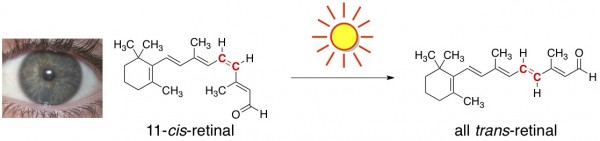
Light Causes Chemical Reactions in our Eyeballs. Obviously, we need light to see, but it’s less obvious that seeing involves chemical reactions. When light bounces off an object into our eyes, it comes in contact with a particular molecule found in our retinas, aptly named “retinal.” Side note – retinal is a (friendly) aldehyde; hence the “-al” ending to the term retinal. It’s a form of vitamin A.
Light causes one particular carbon-carbon double bond in retinal to switch its configuration (from cis to trans), changing the overall geometry of the molecule. This sets off a cascade of chemical reactions that results in a signal to the brain, which is interpreted as vision.
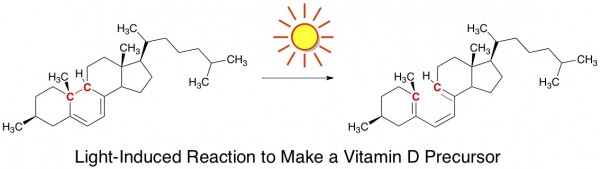
Light Causes Good Chemical Reactions in Our Skin. One of the vitamins we can’t do without is vitamin D. A key step in its synthesis within our bodies involves a light-induced bond breakage. For those of you keeping score, this is a 6 electron ring-opening electrocyclic reaction.
The only way for this step to occur naturally is by skin exposure to sunlight. Prolonged sun time isn’t necessary, but many regions of the worldcough cough michigan get so little sunlight during the winter that vitamin D deficiency can become a legitimate concern. Fortunately, one can take vitamin D supplements during such times, if needed.
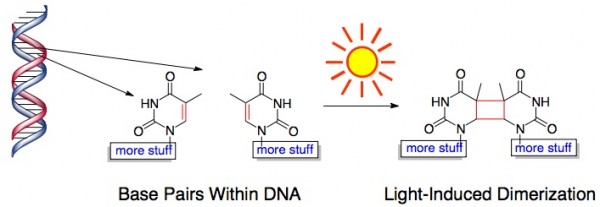
And Light Causes Bad Chemical Reactions in Our Skin. Sunlight, particularly ultraviolet light, can damage the DNA in our skin by triggering portions of DNA to fuse together. This is bad news for our skin cells because that chunk of DNA code is no longer readable. This light-induced chemical reaction looks something like this:
Mild DNA damage by UV light triggers the production of melanin – that dark pigment responsible for a (natural) tan. Melanin acts as a kind of shield for the skin – it absorbs light (as evidenced by the fact that it has a dark color) and prevents UV light from getting through the top layers of skin.
I shall now conclude with a picture reminiscent of the song that has been stuck in my head the entire time I’ve been writing this post.




Great post, and a nice way to usher in spring! Whatever you do, though, please don’t tell me what your Big Bird-related earworm is.
Thanks… yeah you don’t want it in your head. I’m pretty sure I was humming it while running columns today.
How is such a thing possible? Your mother didn’t let you watch Sesame Street!
“Sunny days…chasing the…clouds away…” 🙂
The article makes me wonder if a certain night owl of my acquaintance is that way partly due to gazing at a bright computer monitor late after sunset. As a sequitur to the non-sequitur, song that was evoked in my head at the sight of Big Bird was “Itsy Bitsy Spider.” The only logical explanation that comes to mind is that I recently saw an ad on a business channel for Spyder ETFs and that song was played in connection with an accompanying spider animation and both that song and Big Bird are child stuff.