Consider this an unscheduled footnote to part 2 of the ongoing 3 part series on navigating the language of chemicals. Read an introduction, Part 1, and Part 2 if you wish; Part 3 should appear on Monday.
 While reading about the presence of formaldehyde in hair products, I’ve stumbled across descriptions of a few products that proudly claim to be not only formaldehyde free, but free of any “hyde” or any chemical in the “hyde” family. Though this phrase is relatively harmless, I do find the wording a teensy bit unfair to some of the friendly aldehydes out there. So just to set the record straight, here is why you shouldn’t hate hydes just because they’re hydes.
While reading about the presence of formaldehyde in hair products, I’ve stumbled across descriptions of a few products that proudly claim to be not only formaldehyde free, but free of any “hyde” or any chemical in the “hyde” family. Though this phrase is relatively harmless, I do find the wording a teensy bit unfair to some of the friendly aldehydes out there. So just to set the record straight, here is why you shouldn’t hate hydes just because they’re hydes.
What is a “hyde”?
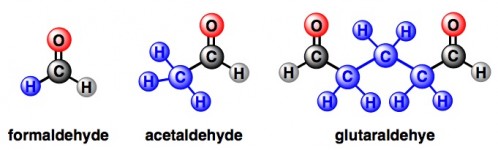
This nickname is clearly referring to chemicals whose names end in the suffix “-hyde”. Formaldehyde, acetaldehyde, and glutaraldehyde are examples that can probably be found in various hair products. More rightly, as you might notice, the complete suffix is “-aldehyde”.
An aldehyde is a type of organiccarbon-based molecule. Specifically, it’s one that contains (somewhere) a set of three particular atoms – carbon (C), oxygen (O), and hydrogen (H) arranged just so:
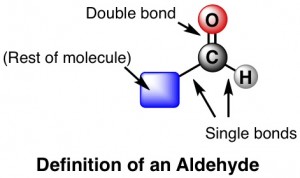
Formaldehyde is the simplest aldehyde – the rest of the molecule besides the requisite CHO piece is just one tiny hydrogen. Acetaldehyde is not much more complicated. And glutaraldehyde is really into the whole aldehyde thing because it has not one, but two sets of CHO groups.
“Hydes” aren’t categorically bad.
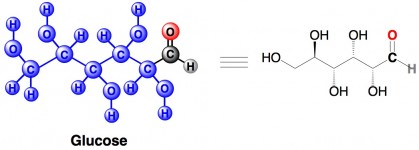
There is a time and a place for formaldehyde, but significant quantities of it don’t really belong in your beverages, on your skin, or in your lungs. So you could loosely say that formaldehyde is bad. On the other hand, everyone knows and loves another aldehyde – glucose.* Glucose is a type of sugar, and it makes up one half of sucrose (table sugar).
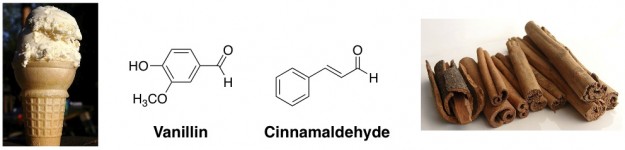
Mix glucose with the two aldehydes below, and you’re well on your way to a dessert! As their names imply, vanillin is the primary flavor/scent component in vanilla extract, and cinnamaldehyde is largely responsible for the taste of cinnamon.
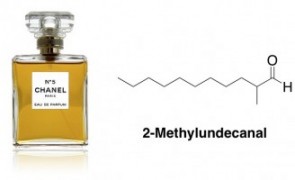
In general, aldehydes tend to have nice odors and flavors, so they are quite popular in perfumes and flavorings. An example is a molecule responsible for the signature scent of Chanel No. 5 – 2-methylundecanal. This aldehyde is an example of one of those unfortunate chemicals that doesn’t have a pleasant-sounding name, despite the fact that it is “natural” (it comes from the peel of kumquats, according to Wiki).
Conclusion – Most types of profiling are risky (racial, gender, etc.) – functional groupthe CHO group is one of many 'functional groups' that can be found in organic molecules profiling is no exception.


But glucose is a ring, 5 carbons, 4 with an OH group, one with a CH2OH, and an oxygen tying them into the ring. I’m not sure what molecule you have there, but I’m pretty sure it’s not glucose.
That said, I think that -hydes DO get a bad rap. And who knew that Chanel #5 comes from kumquats?? 🙂
Nope, it definitely is glucose. It’s shown in the open chain form. You’re probably used to seeing it drawn as the ring form, but in truth it exists as an equilibrium between the open chain and cyclic form (mostly pyranose or 6-membered ring). To be fair, the equilibrium does largely favor the ring rather than straight-chain. See this site for a bit of discussion about the topic. Thanks for the careful reading though! : )
Hey again, I enjoyed reading this but you’ve accidentally labelled 2-methylundecanal as 2-methylundecanol in your diagram. Keep blogging Sharon: I love reading them.
Yikes thanks for the catch! I have corrected it in the picture. (Anyone else reading – he is not being nitpicky, that little “a” means something entirely different than the “o”. The molecule spelled with an “o” isn’t even a “hyde”! If you do that on an organic chem exam you will lose points!!)
Anyway, thanks Tom and I’m glad you enjoy reading this thing.
Great post! Nice accessible overview of the aldehyde functional group as well. Keep up the fight against chemophobia!
The question that jumps out at me immediately is: does every Hyde have a corresponding Jekyll?