HIV Particles. Photo credit PhD Dre at en.wikipedia.
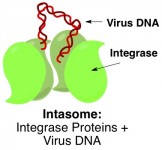
Retroviruses such as HIV work by integrating their genetic code into the DNA of a hostsuch as a person or animal (see part 1). Scientists have known for a while that a certain virus protein, called integrase, is somehow responsible for fusing virus and host DNA together. Four of these integrase molecules cluster around the loose ends of virus DNA. This entire complex of proteins and virus DNA – called an intasome – then captures host DNA, snips it open, and glues the virus DNA ends to the freshly cut host DNA ends.
Back at the beginning of this year, Cherepanov and coworkers published the three-dimensional X-ray crystal structure (see part 2) of an entire intasome comprising an integrase tetramertetramer refers to there being four molecules of integrase working together latched onto the ends of virus DNA. Now they have taken it one step further and obtained a crystal structure of the intasome in the act of snipping open host DNA.
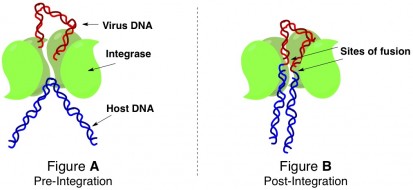
The intasome that the authors crystallized was from a nonpathogenicdoesn’t actually cause any diseases retrovirus called the prototype foamy virus. By altering the conditions of the surrounding media, they were able to grow crystals of the intasome in the process of fusing virus and host DNA at two different stages of the event: the intasome pinning a piece of host DNA in position to cut it open (Figure A below), and the intasome complexed with the host/virus DNA hybrid after DNA strand fusion had occurred (Figure B below).
The structure of the integrase protein tetramer is such that there is a groove right down the middle of the cluster. The ends of the virus DNA are held inside that groove by interactions between certain amino acidsbuilding blocks of proteins of the integrases and specific asking to bethe strain of being bent weakens the strand snipped open. After virus integration has occurred, the complex looks like shown in Figure B. Host DNA has been cut right through (both strands of the double helix have been cut), and one end of the virus DNA has been fused to each segment.
The three-dimensional structural information elucidated by these crystal structures could enable researchers in the future to design drugs that will inhibit the intasome of pathogenic viruses like HIV before it gets a chance to latch onto host DNA. Alternatively, bioengineers can think about designing a mutant nonpathogenic virus with a special intasome that would be much more discriminate about host DNA sites to cut open. Such an engineered virus could be used as a Trojan horse to introduce special DNA sequences into specific DNA sites of bacteria that could convert normal bacteria into, say, bacteria that produce gasoline.

