 If you’ve ever stared pensively into a mug of beerunless it was Guinness, then you might be picturing N2 bubbles, then you probably have a good mental picture of what CO2 bubbles look like. Tiny little things that wiggle their way up or cling to the sides of the glass… nothing terribly exciting, right?
If you’ve ever stared pensively into a mug of beerunless it was Guinness, then you might be picturing N2 bubbles, then you probably have a good mental picture of what CO2 bubbles look like. Tiny little things that wiggle their way up or cling to the sides of the glass… nothing terribly exciting, right?
Well, a recent study in Angewandte Chemie demonstrates that there is waaay more than meets the eye with these bubbles. Unlike nitrogen (the main constituent of air), carbon dioxide does some unusual things when dissolved in water. For one, it dissolves really well – you can pack at a lot of carbonation into a bubbly beverage. Secondly, CO2 actually reacts with water a little bit.
Because CO2 bubbles seem to be special, researchers Dagastine and coworkers decided to investigate their behavior in depth. They created microscopic bubbles of CO2 in water at different pH values7 = neutral, anything lower is acidic and higher is basic (different levels of acidity), and then examined how easily the CO2 bubbles would coalesce, or squish together to become bigger bubbles.
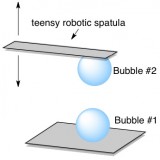
To do this, they picked up one microscopic bubble with a tiny robotic spatula and slowwwly moved it toward another bubble that they’d placed on a glass plate. (Just to make sure we’re on the same page, these bubbles are actually thin spherical films of water filled with CO2.) If the microscopic robotic spatula ever sensed even a tiny bit of repulsion between the two bubbles, it stopped moving. If no repulsion happened, the spatula continued to bring its bubble closer until the two bubbles coalesced into one.
They then repeated this experiment with some different types of bubbles – nitrogen, argon, and air – also produced in water at different pH values. Interestingly, whereas nitrogen or argon bubbles were happy to coalesce within a broad range of pH values (pH 3 to pH 7), CO2 bubbles were much pickier and would only join up with each other if the pH was just right (just barely above pH 6). At any lower pHand at higher pH CO2 couldn't exist as CO2, so no bubbles, the CO2 bubbles would repel each other.
At this point, it is interesting to note that soft drinks and beer have relatively low pHs (they’re kind of acidic) – significantly lower than pH 6. This means that CO2 bubbles in beer or Coke want to stay away from each other. That’s a good thing, because your beverage would go flat rather quickly if all the microscopic bubbles just joined up right away to make one gigantic bubble. (The low pH is also required for/an effect of CO2 being dissolved.)
So how does a bubble decide whether or not to coalesce with another bubble? You see, each bubble has two opposing forces acting on it at any given time. You can think of these forces as an angel and a devil whispering conflicting messages over the bubble’s shoulder. The first force, called a van der Waals force (we’ll say that one’s the angel…?) says, “Go on, be friendly. Join up with that other bubble.” Meanwhile, the devil – a force called electrostatic repulsion – says “That other bubble sucks. Stay away from it.”
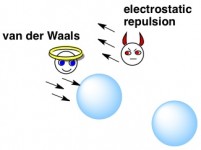
Whether the angel or devil wins depends on the pH of the bubble’s original solution, as well as what else might be on the bubbles’ surfaces.
The van der Waals force is a constant weak attraction between one bubble’s surface and the other’s, and relates to random fleeting partial charges (+ or – charges) among uncharged molecules (due to the random movement of electrons, you could say). This force is real, but weak, and it can’t be felt until the bubbles get close enough together.
The electrostatic repulsion force – the one that pushes bubbles apart – occurs when the bubbles’ surfaces have an overall net + or – charge, which is partly dependent on the pH and the content of the solution they’re in. Two bubbles that both have a negatively charged surface, for example, will not want to come very close to each other.
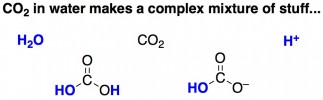
For CO2 bubbles, it seems that electrostatic repulsions are almost always stronger than van der Waals attractive forces. The researchers propose that this is due to CO2-related molecules adsorbed onto the surface of the bubble. Unlike nitrogen or argon, when CO2 dissolves in water it reacts to form a mish-mash of molecules, some of which are charged and can coat the exterior of a bubble, bringing about changes to the bubble’s surface charge.
The researchers point out that the odd behavior of CO2 should be taken into account when studying air bubbles too (if you’re someone inclined to do that…), since CO2 is one of the minor constituents of air. And in fact, the behavior of air bubbles was sort of intermediate between that of N2 and CO2.
But please, don’t let thoughts about bubble mechanics spoil your next trance-like gaze into a mug of beer.
Citation:
Tabor, R., Chan, D., Grieser, F., & Dagastine, R. (2011). Anomalous Stability of Carbon Dioxide in pH-Controlled Bubble Coalescence Angewandte Chemie International Edition DOI: 10.1002/anie.201006552


Mmmmmm…..the chemistry of beer. 🙂
Kind of related – in an article explaining the “bends” and why it can be avoided by ascending from a lengthy deep water dive slowly, the writer says the slow ascent avoids the symptoms of “the bends” the same way bubbling of beer is avoided by opening the cap on the beer bottle slowly. After reading this post about angelic CO2 bubbles (I’m a mug half full perspective person) it seems a beer bottle opening is not the best analogy for “the bends.” Since “the bends” is involving nitrogen bubbles and not CO2 bubbles (which is what is in beer) – the painful symptoms may be due in part to the nitrogen bubbles coalescing to become bigger and therefore more painful?
And, of course, if we add a mint Mento into the soda, the bubbles are attracted to the uneven surface (by VdW forces? Dipole interactions? Hm.), and we get MANY larger bubbles forming at once… which is fun. 🙂
Hi Sharon
This is an interesting article – something I will be pondering when I next open a beer. Would be interesting to know how the first beer makers got it right? by trial and error, I would suppose.