 The cast of the strangely popular TV show Jersey Shore are known for their extreme obsession with tanning. Earlier this year, Snooki switched from tanning beds to using spray-on tans, citing Obama’s tanning tax and “friggin’ cancer”. Unlike UV light, sunless tans don’t stimulate the production of the brown pigment melanin. Nor are they merely spraying on paint – the active ingredient is actually colorless. So what kind of chemistry is going on on Snooki’s skin?
The cast of the strangely popular TV show Jersey Shore are known for their extreme obsession with tanning. Earlier this year, Snooki switched from tanning beds to using spray-on tans, citing Obama’s tanning tax and “friggin’ cancer”. Unlike UV light, sunless tans don’t stimulate the production of the brown pigment melanin. Nor are they merely spraying on paint – the active ingredient is actually colorless. So what kind of chemistry is going on on Snooki’s skin?
Well, it’s actually a kind of complicated sequence of reactions that aren’t fully characterized beyond the first few steps. The active ingredient in most sunless tanners is dihydroxyacetone (DHA), a very simple carbohydrate. DHA reacts with amino acids in dead cells on the surface of the skin. A molecule of DHA combines with an amino acid; next a molecule of water is lost, followed by a rearrangement (ref). The resultant molecule then goes on to combine with other similar molecules to form polymers, which have a brownish color (polymers are really big molecules, and big molecules have a tendency to be colored).
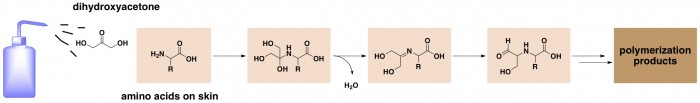
 This reaction on the skin is actually very similar to a key reaction in food chemistry – the Maillard reaction – which is responsible for many types of food browning. This reaction can occur in any food that contains both sugars and amino acids, and usually requires heat to get it started. In the Maillard reaction, a sugar like glucose (instead of DHA) reacts with amino acids to produce polymers and small molecules responsible for the colors and flavors of foods like roasted coffee, brioche, and malted barley (according to Wikipedia).
This reaction on the skin is actually very similar to a key reaction in food chemistry – the Maillard reaction – which is responsible for many types of food browning. This reaction can occur in any food that contains both sugars and amino acids, and usually requires heat to get it started. In the Maillard reaction, a sugar like glucose (instead of DHA) reacts with amino acids to produce polymers and small molecules responsible for the colors and flavors of foods like roasted coffee, brioche, and malted barley (according to Wikipedia).


I say skip the tanning booth and just sit in the oven for awhile. A nice brown crust will surely develop!