Scientists have discovered a new way to build nanoscale towers. Each tower is only a few nanometersThe thickness of a piece of paper is about 100,000 nanometers wide and a couple thousand nanometers tall. For scientists who work with nanomaterials, it is relatively easy to build tiny tunnels (horizontal structures), but building towers (vertical structures) has been a much bigger challenge. Nevertheless, ways to build vertical towers are in high demand because these nano-towers have potential applications for optical devices like solar cells.
The trick that Seki and coworkers developed to build these tiny towers relies on a phenomenon called π-π stackingPronounced pi-pi stacking; also called π-π interactions. To understand π-π stacking, which is a type of intramolecular interaction, we first need to take a couple of steps back.
Brief Tutorial in Intramolecular Interactions. A basic tenet of chemistry is that atoms form bonds with each other to make molecules. A more subtle phenomenon is that of intramolecular interactions. These are favorable interactions between two or more molecules that result in these molecules “sticking” to each other without actually forming any chemical bonds. For example, water molecules have relatively strong intramolecular interactions with each other; for this reason, it takes a lot of heat (energy) to boil water, or force the molecules apart enough to become a gas rather than a liquid.
intramolecular interactions with each other; for this reason, it takes a lot of heat (energy) to boil water, or force the molecules apart enough to become a gas rather than a liquid.
There are different kinds of intramolecular interactions. One of the less common types is π-π stacking. This interaction is a bit complex to explain, but suffice to say that a π-π interaction occurs between two molecules that both have portions that look kind of like benzene. These benzene-like portions are sticky to each other; because of this stickiness, molecules try to line up with each other so that there is maximum surface area “touching”.
π-π stacking. This interaction is a bit complex to explain, but suffice to say that a π-π interaction occurs between two molecules that both have portions that look kind of like benzene. These benzene-like portions are sticky to each other; because of this stickiness, molecules try to line up with each other so that there is maximum surface area “touching”.
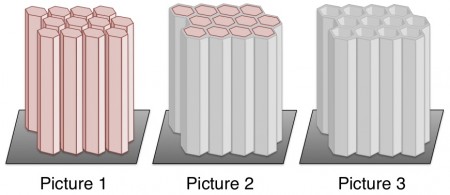
π-π Stacking To Build Towers. Seki and coworkers found that they could take advantage of π-π stacking to build nanoscale towers. π-π interactions enabled the researchers to build tall stacks of organic molecules (Picture 1); they then filled in the space around the towers with silicaGlass is made of silica (Picture 2). Finally, they got rid of the organic molecules, leaving behind hollow nanoscale towers made of sturdy silica (Picture 3).
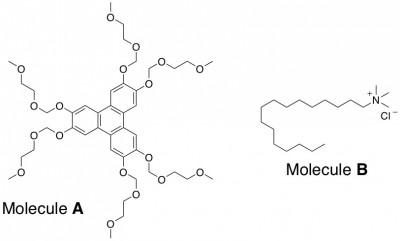
How They Did It: Choosing the Right Organic Molecule. The organic molecule that the researchers chose to use, labeled as A below, has some important characteristics. First, this molecule has four benzene rings in it, so there is a lot of potential for π-π interactions between molecules. Secondly, A is nearly flat like a dinner plate, so it can stack up much better than a round or odd-shaped molecule could. The researchers demonstrated the importance of the π-π stacking ability of A by trying to build towers using a rod-shaped molecule, B, that can’t participate in π-π stacking (no benzene-like rings). B didn’t form towers; the molecules just randomly aligned themselves on the surface.
How They Did It: Choosing the Right Surface. Importantly, the researchers built the stacks of A on top of thin sheets of graphiteSpecifically, highly oriented pyrolytic graphite. Graphite, like A, is also able to participate in π-π stacking. For this reason, when molecules of A are poured onto graphite, the first molecules that hit the graphite surface want to lie completely flat to maximize the surface area that is touching the graphite. The next molecules of A then stack up on top of each other. When the researchers instead tried using a surface made of silicon, a material that can’t participate in π-π interactions, the molecules of A didn’t build towers, and instead stacked horizontally like Oreos in a box.
For this reason, when molecules of A are poured onto graphite, the first molecules that hit the graphite surface want to lie completely flat to maximize the surface area that is touching the graphite. The next molecules of A then stack up on top of each other. When the researchers instead tried using a surface made of silicon, a material that can’t participate in π-π interactions, the molecules of A didn’t build towers, and instead stacked horizontally like Oreos in a box.
What Next? You could imagine that towers with different widths could be formed by using bigger or smaller disk shaped molecules instead of A. (Maybe a pizzane?!) With tunable tower width, structures can be built and studied for a variety of applications, most notably organic solar cells.

