Here’s a fun tip. If you get bored reading ASAPsAs Soon As Publishable - very recent articles, try doing the exact opposite. What was seen as cutting-edge research in the earliest issues of the Journal of the American Chemical Society makes for quite a different read than today’s articles.
JACSJournal of the American Chemical Society (est. 1879) has been around almost as long as Mendeleev’s periodic table (est. 1869), and the articles from the 1880s give us a pretty good idea of what was going on at the dawn of modern chemistry. Apparently, chemists back then were fond of taking everyday things and figuring out what was in them. Methods of analysis typically involved burning things, boiling things, and mixing things with strong acids and bases. Other hot research trends included discovering new elements and developing new procedures for weighing things.
Let’s take a look at the sort of research that made it into JACS back in the day.
“Note On a Sample of Old Butter” – 1887
This author’s wife must have been very patient, because she put up with a stinking tin of rancid butter on the window sill for two years. A. A. Breneman describes his procedure of leaving a tin box full of butter out, with periodic sniffing to assess changes in the “rancid odor”. Fortunately, the smell disappeared after a few months. By the end of two years, the mass of was-butter had become “tallow-like and opaque”, and some granules had crystallized out. Breneman supposes that these “are probably stearine,” and leaves it at that. Voila, a JACS paper! Strengths of this research include that it is easily reproducible by really anyone.
“On the Composition of Elephants Milk” – 1881
I really appreciated the level of irrelevant (but amusing) detail in this publication. Charles Doremus describes seeing an ad about a baby elephant (presumably born in the city zoo) and wondering what was in elephant milk. (The thoughts of a true scientist.) He methodically discusses the difficulty in obtaining milk from the nursing mother, and notes that her name was Hebe, and the baby was named America. His milk analysis method seems to mostly involve just looking at it; though he also smelled and tasted it, and did a little evaporating and extracting with base and ether. Key results include that the milk was 66.697% water (apparently that many decimal points was necessary and considered accurate) and 22.070% fat. (The rest of the material was “casein, sugar, and ash”.) Doremus was generous enough to include a handy quick-reference table to post on your refrigerator icebox, comparing elephant milk to several other types of milk including from camels, mares, hippopotamus, and women. His conclusions are summarized by the following statement:
“From whatever standpoint, therefore, we view the lacteal product of these four-footed giants, we are fully warranted in ascribing to it not only extreme richness, but also great delicacy of flavor.”
“On the Probable Occurrence of Norwegium in American Lead” – 1880
Huh? I’ll admit that just to be sure, I checked my periodic table to see if this was tucked in with the actinides where I might not have noticed it before. But no, no norwegium. Apparently a guy named Tellef Dahll “discovered” norwegium in a mine in 1878. A couple years later, Geo. A. Prochazka was pretty sure he’d found the same thing in American lead, and published his analysis in JACS. Prochazka describes norwegium as being pretty similar to bismuth (which is a legit element). I hunted around a bit trying to figure out what this norwegium really was, but Google didn’t give me many answers. A few search results seem to loosely link it to hafnium, but nothing solid really panned out. However, just by comparing properties, the most similar element to the “norwegium” described by Prochazka is polonium, which was officially discovered by Marie Curie in 1898.
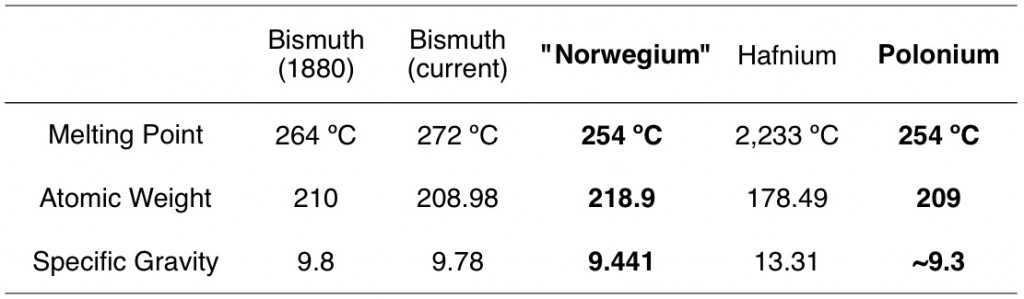 If norwegium = polonium, I hope Dahll and Prochazka weren’t analyzing this one by taste like it was elephant milk. Polonium is radioactive; in fact, one of the less stable isotopes, 210Po was used to assasinate the Russian politician Alexander Litvinenko in 2006. Nevertheless, I’m not sure we can trust the norwegium properties described by Prochazka, as his citation of Dahll seems to be of questionable origin. Dahll’s discovery of norwegium is mentioned a couple of times in The Manufacturer and Builder in 1879 and 1880, but this source reports a different mass (146) and a different melting point (350 ºC) than Prochazka cites. Either way, it’s amusing that nobody ever felt the need to publish a correction or update to this article, despite that fact that norwegium doesn’t exist.
If norwegium = polonium, I hope Dahll and Prochazka weren’t analyzing this one by taste like it was elephant milk. Polonium is radioactive; in fact, one of the less stable isotopes, 210Po was used to assasinate the Russian politician Alexander Litvinenko in 2006. Nevertheless, I’m not sure we can trust the norwegium properties described by Prochazka, as his citation of Dahll seems to be of questionable origin. Dahll’s discovery of norwegium is mentioned a couple of times in The Manufacturer and Builder in 1879 and 1880, but this source reports a different mass (146) and a different melting point (350 ºC) than Prochazka cites. Either way, it’s amusing that nobody ever felt the need to publish a correction or update to this article, despite that fact that norwegium doesn’t exist.


