A “new” life form was documented in a Science article released yesterday. OK, so it’s not exactly alien life: it was found in California (Earth) and, technically, its unusual characteristics are kind of due to human interference. Regardless, these little microbes certainly sound like science fiction.
 As a rule, all life forms require the element phosphorus to make key biological molecules like DNA. However, these eccentric bacteria are somehow capable of using arsenic as a phosphorus substitute. Derek Lowe at In the Pipeline has already done a stellar job of summarizing the Science paper’s findings, but the basic idea is that some scientists (astrobiologists from NASA to be specific!) found a type of bacteria that seemed a little more tolerant of arsenic than most. They isolated these bacteria and gradually fed them higher and higher doses of arsenic while simultaneously feeding them less and less phosphorus. The bacteria adapted to their increasingly toxic conditions, and by the end of the experiment, they were able to subsist on arsenic-based food without being given any phosphorus at all. If you’re thinking that arsenic is a poison, you’re right – it usually isSo don’t try this on your pet chinchilla.
As a rule, all life forms require the element phosphorus to make key biological molecules like DNA. However, these eccentric bacteria are somehow capable of using arsenic as a phosphorus substitute. Derek Lowe at In the Pipeline has already done a stellar job of summarizing the Science paper’s findings, but the basic idea is that some scientists (astrobiologists from NASA to be specific!) found a type of bacteria that seemed a little more tolerant of arsenic than most. They isolated these bacteria and gradually fed them higher and higher doses of arsenic while simultaneously feeding them less and less phosphorus. The bacteria adapted to their increasingly toxic conditions, and by the end of the experiment, they were able to subsist on arsenic-based food without being given any phosphorus at all. If you’re thinking that arsenic is a poison, you’re right – it usually isSo don’t try this on your pet chinchilla.

A "Horta" from Star Trek (credit Wikimedia)
From time to time, science fiction has featured alien life forms that are made of and consume unusual elements. The first example that comes to mind is a hypothetical silicon-based life form on Star TrekI am/was never a Trekkie but my dad was/is.. Whereas normal life (on Earth) uses carbon as the primary element for all biological molecules, it has been fantasized and hypothesized that an alternative life form could perhaps use silicon everywhere one would normally use carbon. Why silicon for the Star Trek creature? And why arsenic for the weirdo bacteria?
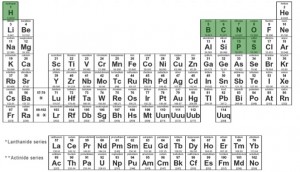
The answer lies in the periodic table. On first pass, this chart is an intimidating beast, with over 100 different elements represented. It is reassuring, however, that only seven of those elements are highly featured in biological life (highlighted in green, below).
By knowing the simple spatial location of any given element on the chart, you can gather a lot of information about that element’s properties. One of the most informative features of the chart, and the one relevant to silicon- or arsenic-based life forms, is the vertical columns. In chemistry lingo, the columns are called groups. Two atoms that are in the same group have the same set-up for their electrons – at least, for the atoms’ “outermost” electrons (which are the ones usually involved in all the action). This can’t be said for two atoms in different groups.
For example, oxygen and sulfur are in the same group. There are two layers of electrons around oxygen’s nucleus, and the outermost layer has six electrons in it. Oxygen is depicted below in cartoon form, in which the electrons look kind of planets orbiting the sun (different “layers” = different “orbits”). Sulfur is a fatter atom – it has three layers of electrons around its nucleus. Nevertheless, sulfur’s outermost electron layer looks just like oxygen’s, with six electrons. For comparison, boron is also shown below. Boron is in a different group entirely, and its outermost layer comprises just three electrons.
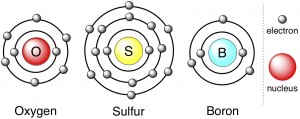
Not surprisingly, the chemical behavior of boron is quite a bit different than sulfur or oxygen, particularly in the way it makes bonds. Bonds are formed using electrons from atoms’ outermost layer. Thus, boron has to take a different approach to bonding than sulfur or oxygen do, since it has fewer electrons to work with. Conversely, sulfur and oxygen tend to behave similarly when it comes to bonding.
![]() So now if you look closely back at the periodic table, you’ll notice that silicon (Si) is directly below carbon (C). Indeed, silicon’s bonding properties are in many ways similar to carbon’s. Likewise, arsenic (As) shares traits with phosphorus (P) since these two are also in a group together. Thus it’s not completely unreasonable to think that arsenic could sub in for phosphorus in biological molecules, or that silicon could take the place of carbon.
So now if you look closely back at the periodic table, you’ll notice that silicon (Si) is directly below carbon (C). Indeed, silicon’s bonding properties are in many ways similar to carbon’s. Likewise, arsenic (As) shares traits with phosphorus (P) since these two are also in a group together. Thus it’s not completely unreasonable to think that arsenic could sub in for phosphorus in biological molecules, or that silicon could take the place of carbon.
The authors of the arsenic-eating-bacteria article provide a lot evidence supporting the idea that the bacteria have replaced almost all of the phosphorus in their “bodies” with arsenic. They don’t have absolute proof yet for phosphorus being incorporated into DNA (an X-ray crystal structure would be lovely here, as Derek mentions), but that’s certainly in the works.