 Having no reason to do my hair since I’m in lab all day Having naturally straight hair, I don’t usually pay attention to trendy methods of hair-straightening. Until yesterday, that is, when a colleague pointed out a Fashionista article to me about the “Brazilian Blowout.” For those of you who don’t know what this Blowout is (like me about 24 hours ago), it’s a “keratin treatment” popular with celebrities that will give you “smooth, healthy, frizz-free hair with radiant shine!”
Having no reason to do my hair since I’m in lab all day Having naturally straight hair, I don’t usually pay attention to trendy methods of hair-straightening. Until yesterday, that is, when a colleague pointed out a Fashionista article to me about the “Brazilian Blowout.” For those of you who don’t know what this Blowout is (like me about 24 hours ago), it’s a “keratin treatment” popular with celebrities that will give you “smooth, healthy, frizz-free hair with radiant shine!”
Currently the Brazilian Blowout is under intense scrutiny because OSHAOccupational Safety and Health Administration and Health Canada recently found levels of formaldehyde up to 12% in the Brazilian Blowout hair product. This is 60 times the recommended “safe” levels of formaldehyde for hair products (0.2%). Brazilian Blowout (the company), however, claims that their product doesn’t contain formaldehyde. They’ve been in a back-and-forth exchange with OSHA, and one of their rebuttals to the health and safety officials is particularly funny.
Brazilian Blowout claimed that OSHA’s reports were unreliable because they tested for methylene glycol, not formaldehdye. Now for those of you who don’t know chemistry nomenclature, methylene glycol is basically just another name for formaldehyde. If Brazilian Blowout puts “methylene glycol” into their hair product solution, then, yes, their formula contains formaldehyde. (You’d think Brazilian Blowout would have had their lawyers/scientists check on that before they blew up at OSHA… or before they marketed their product as “Formaldehyde Free”).
The news media is having a tough time with this concept. Says the Fashionista article author:
I decided to consult an expert. I spoke to a PhD organic chemist with more than 20 years of experience in the pharmaceutical industry about the chemical properties of formaldehyde. After an informative lesson on carbonyl structure, he said, “The long and short of it is that methylene glycol equals formaldehyde, period, get over it, done, move on.”
Unless you’ve taken/understood organic chemistry, it probably won’t be immediately obvious why methylene glycol = formaldehyde. You see, methylene glycol is really just the name for formaldehyde dissolved in water. Here’s an explanation that takes into account chemistry terms that most people are familiar with (even if they don’t realize it).
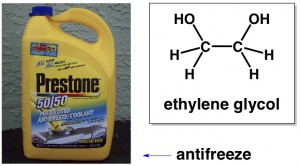 You’ve probably heard of ethylene glycol. It’s antifreeze, like what you might put in your car. If you look at its chemical structure, you will see two “OH” groups attached to carbons. Whenever you have two OH groups like that, you can call the molecule a “glycol”. In organic chemistry naming, the prefix “eth” always indicates that there are two carbons in whatever molecule or section of a molecule that is being named. In this case, the name “ethylene” reflects the fact that there are a total of two carbons in the molecule.
You’ve probably heard of ethylene glycol. It’s antifreeze, like what you might put in your car. If you look at its chemical structure, you will see two “OH” groups attached to carbons. Whenever you have two OH groups like that, you can call the molecule a “glycol”. In organic chemistry naming, the prefix “eth” always indicates that there are two carbons in whatever molecule or section of a molecule that is being named. In this case, the name “ethylene” reflects the fact that there are a total of two carbons in the molecule.
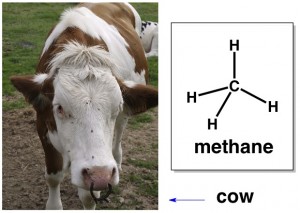 You’ve also certainly heard of methane. It’s a greenhouse gas, cows are major producers, etc. If you look at methane’s chemical structure, you’ll notice that there is only one carbon. The prefix “meth” is always used in organic chemistry to indicates that there is just one carbon in the molecule or section of a molecule. (The street drug “meth” has its nickname derived from a particular portion of the drug molecule that consists of a single carbon.)
You’ve also certainly heard of methane. It’s a greenhouse gas, cows are major producers, etc. If you look at methane’s chemical structure, you’ll notice that there is only one carbon. The prefix “meth” is always used in organic chemistry to indicates that there is just one carbon in the molecule or section of a molecule. (The street drug “meth” has its nickname derived from a particular portion of the drug molecule that consists of a single carbon.)
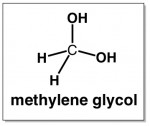
So, if we take the prefix “meth” and the word “glycol” and try to turn it into a structure like ethylene glycol but with only one carbon, it would have to look like this:
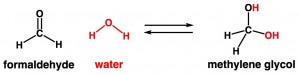
Finally, let’s look at formaldehyde. Formaldehyde’s structure is shown below, but the instant you mix it with water, it combines with water to make methylene glycol. Water is colored red in the scheme below so you can see where the atoms from water stick onto the formaldehyde. Importantly, the reverse reaction happens really easily too, in which methylene glycol turns into formaldehyde (+water). Individual molecules of formaldehyde quickly turn into methylene glycol, but then quickly turn back into formaldehyde, then back into methylene glycol, and back and forth forever. Methylene glycol can be called the “hydrate” of formaldehyde. Like it sounds, a hydrate is basically a molecule’s structure when it has “soaked up” water. Since water is everywhere (including hair products, the air, and our bodies), a test for methylene glycol means the same thing as a test for formaldehyde.
I will leave you with a final quote from the Fashionista article. I’m all for non-chemists being impressed by the wonders of science… so I almost don’t want the author of this quote to ever figure out that she’s talking about a rather fundamental property of chemistry:
[The PhD chemist with 20 years of experience] also told me that there are chemicals that can be put into solution that when heated, turn into formaldehyde quickly. So a chemical that is not technically formaldehyde in solution, can turn into formaldehyde. The miracle of modern chemistry.


If you’d like to read a “hair-raising” look at the effect of nasty chemicals on your head, look at the Autobiography of Malcom X. African Americans often used lye to straighten their hair, and Mr. X once had to use a toilet to wash the caustic mess off his head.
Thanks for the tip and the pun, Eric! NaOH in the hair sounds like a disaster waiting to happen…
Sodium Hydroxide is still the active ingredient in many of the perms and straighteners we use today. They’re called alkaline or cold wave perm solutions.
Never would have thunk I would find this so indspiensblae.
I’ve been alerted by a Brazilian Blowout spokesperson to read a press release from yesterday (see below) describing further tests by OSHA of salon air for formaldehyde during the the application of a Brazilian Blowout treatment. The press release claims that the air levels are within a safe range. I draw no conclusions at this time.
http://www.businesswire.com/news/home/20101130007194/en/Oregon-OSHA-Confirms-Brazilian-Blowout-Safe
Strong work, Sharon. I of the frizzy, curly mop am all too familiar with straightening techniques. But I’m too cheap to shell out for chemical straightening. FYI, that press release isn’t on Oregon OSHA’s site: http://www.orosha.org/admin/newsrelease/2010/news_2010.html
And here’s the full report that the press release quotes from: http://www.orosha.org/pdf/Final_Hair_Smoothing_Report.pdf
No one ever seems to mention that the BB PASSED the test:(the following is from the OSHA report. I haven’t seen this much erroneous expert opinion since I read about the Salem witch trails.
Oregon OSHA also conducted air monitoring during treatments using the Brazilian Blowout
smoothing product at seven different salons where a single treatment was conducted over the
course of the day. The 8-hour average exposures ranged from a low of 0.006 parts per million
(ppm) to 0.33 ppm. These compare to a permissible exposure limit of 0.75 ppm. Although it
was not exceeded for any of these stylists, it should be noted that multiple treatments would
increase the daily average significantly.
I noticed that as well Tony. Why, in all these articles, do they not mention that OSHA retested and that BB passed with FLYING COLORS? I think there is a bigger force behind all of this. A huge beauty company, the biggest in the worLd in fact, whO is planning on coming out with theiR own product vEry soon. Nothing like trAshing the competition…especiaLly since BB is the best one out there.
Thanks Carmen! The OSHA report does clarify a few things. If I had to make an assessment, I’d say the press release is taking a few statements out of context and failing to acknowledge some other important points.
A key point is that in the air tests, each salon only performed one Brazilian Blowout treatment in an entire day. In the following quote from the OSHA report, PEL refers to permissible exposure limit:
“The highest exposure was 66 percent of the action level and 44 percent of the PEL. However, if the same stylist had performed one more comparable two-hour procedure in the course of the same day, the time-weighted-average would likely have been approximately twice as high, putting it well over the action level and at more than 85 percent of the PEL.”
Sharon,
There are so many inconsistencies with this report it’s quite laughable. First, if there was 12% formaldehyde in the product which Oregon OSHA claims, people would be running out of the salon from the fumes. Also, notice how OSHA redid their test and now shows 6% formaldehyde. Inconsistencies all over the place. Lastly, to address your theory that formaldehyde and methlyne glycol are the same is flawed. They are two different chemical elements, with 2 different CAS #s and in 2 different families, alcohol family and the hyde family in terms of chemical structure. Ask any well versed chemist and they will tell you unequivocally that formaledehyde and methlyne glycol ARE NOT THE SAME! Your analogy of putting methlyne glycol + water = formaldehyde is correct, however if you mix 2 parts hydrogen (highly flammable) with 1 part oxygen to create water, it certainly doesn’t mean that water is flammable. I am now highly suspect of OSHA’s findings.
Speaking of inconsistencies, allow me to correct a few things. Formaldehyde and methylene glycol are not elements… They are compounds (aka molecules). Elements are things like carbon (C), nitrogen (N), or oxygen (O). Also, there is no such thing as the ‘hyde’ family. Perhaps you mean aldehyde. Finally, to state that molecules are different because of their CAS# is really a misuse of the CAS system. The system is designed to assign a numerical identifier to every different chemical structure, element, mineral, alloy etc. in the scientific literature. It says nothing about reactivity, only differences in structure or chemical make-up on paper. As many of us know, things on paper are often far different than they are in reality.
Chemistry is often the same, structures written on paper do not adequately reflect how things behave in the real world. Chemists study the reactivity of compounds and molecules under different conditions. The fact that people can draw a different structure for methylene glycol and formaldehyde is irrelevant. What’s important is that the two compounds interconvert rapidly in water, i.e. one does not exist without the other. To suggest that methylene glycol in water exposed to heat will not convert to formaldehyde is a bit like putting a block of ice in a warm room and expecting the floor to stay dry.
A similar phenomenon exists with carbon dioxide (CO2) and water. When dissolved in water a portion of CO2 converts to carbonic acid (H2CO3). Soft drinks for example contain carbonic acid as a result of being carbonated with CO2. This is why soda has an acidic pH and carbonated water gets a slightly sour taste. When a can of soda is opened CO2 escapes and H2CO3 converts to CO2 and escapes as well. This is directly analogous to what happens with methylene glycol and formaldehyde. As it’s heated formaldehyde escapes and methylene glycol is converted to formaldehyde and escapes into the air as well.
As a “well versed” chemist I would caution you and anyone from making health and safety decisions based on differing CAS #’s because it just doesn’t matter. Furthermore, OSHA hires lots of chemists that look out for people’s Safety and Health, so wouldn’t you rather they err on the side of caution?
John2hair, you either didn’t actually read the whole report, or you didn’t understand what it says. It’s actually quite clear in it’s purpose, intent, and conclusions.
I encourage you to take another look. Also take a look at the list of authors and their qualifications. While you’re at it, take a look at the list of PhD reviewers and their qualifications.
I’d really love to know the IP address of john2hair. I’ll bet it leads to a PR firm, but I could be wrong…
I agree. That is the same conclusion I came to after consulting with actual chemists.
@John2hair: Thanks for the comment. Your opinion is shared among many involved in the cosmetics industry, though I would venture that most “well-versed” chemists would disagree. For example, the American Chemistry Council has issued a formal position on the methylene glycol/formaldehyde controversy; they agree that formaldehyde dissolved in water (methylene glycol) should be counted as formaldehyde.
In particular, the analogy of mixing oxygen and hydrogen to make water doesn’t work so well. This reaction can be done, but under quite extreme conditions relative to ambient (e.g., with electrolysis). In contrast, both the forward and reverse reactions of water and formaldehyde happen quite rapidly at room temperature without any special conditions.
I certainly agree with you that, on paper, formaldehyde and methylene glycol are different. But a more relevant analogy might be comparing water with ice. Or salt water compared to salt crystals.
The amount of formaldehyde/methylene glycol in each bottle could probably vary for several reasons. For example, it could be dependent on how long the bottle had been physically open to air, allowing formaldehyde to evaporate out of the bottle. This is exactly why OSHA takes multiple samples; to come up with averages, standard deviations, etc.
thank you for your information. It just shows how companies will try to spin and lie to make a profit at the expense of people. Would not use this treatment until I know it is safe to do so.
Methylene glycol is not formaldehyde. Methylene Glycol is polyhydroxy alcohol and a member of the alcohol family whereas formaldehyde is an Aldehyde. But Methylene glycol can be converted to aldehydes through oxidation process and that is where the formaldehyde comes out.
As a salon owner and stylist, I was doing the Global Keratin Express, which contains Cyprosil, which is some form of “an aldehyde”. One of my friends works at a prominent salon where she did 3-4 Brazilian Blowouts a day. She is now hospitalized for an incurable lung disorder. I am appalled that greed trumps illness. This salon has 7 in the Albuquerque area and plenty of funds to create a ventilated room, provide masks, etc. Did this happen? No. People would not go to a salon if people were sitting around smoking all day long, why is this any different?
I am no longer doing any keratin treatments of any type, as I will not expose my clients nor my stylists to harmful chemicals. Really, people, get a clue! Do you want to be responsible for someone having a lifelong illness or cancer or turkey arm babies, just so you can have shiny Barbie hair or profit off of other peoples vanity? Embrace the hair you have, pull your frizzy head out of your ass and move on. It’s not worth it! Also, everyone is complaining that we are in a recession, and no one has any money, yet women are dumping $150-$500 to be poisoned. Yet they can’t buy gas for their vehicles. I also am never surprised when a government agency lies or when a corporation lies. Trust your gut. If your gut says rape and pillage peoples wallets, then provide a room that will expose only you and your client to toxic chemicals. If your gut says to play it safe and educate your clients on safe products, alternative styling techniques and learning to love the hair they were born with, then go with that!
Bridgette, I totally agree with your comments
It is very hard to convince other people that these chemicals in our salons are so unhealthy for us. They need to be educated about this, that’s the problem. How do we get this out to the public?
As a chemistry layman I make the following analogy:
For the manufacturer of this product to state that this product contains little or no formaldehyde is sort of like a charcoal manfacturer explicitly stating that there is little to no carbon monoxide in their product. True enough for both products but when used in the intended manner, both emit a dangerous substance that the end user should be aware of and take precautions against.
Hi there, just changed into alert to your blog via Google, and found that it is truly informative. I’m going to watch out for brussels. I will appreciate when you proceed this in future. Many people will likely be benefited out of your writing. Cheers!
all i want top know is “does Salerm keratin hair products contain formadelhyde?”