My all-time favorite chemical reaction has to be the SN2. This may sound like a cop-out answer to Rachel Pepling’s question, since the SN2 is arguably the second* simplest organic reaction ever, almost more a mechanistic step than a reaction per se.
 However, its simplicity is deceiving. Our old familiar SN2 reaction is an absolute treasure trove of organic chemistry principles. The details of this reaction exquisitely illustrate nucleophilicity/electrophilicity, stereochemistry, sterics, kinetics, solvent effects, and so very much more. There are ritzy versions of the SN2 (e.g., Mitsunobu) for those of us that want a little extra sophistication. The SN2 can even cure – or cause – cancer (DNA alkylation).
However, its simplicity is deceiving. Our old familiar SN2 reaction is an absolute treasure trove of organic chemistry principles. The details of this reaction exquisitely illustrate nucleophilicity/electrophilicity, stereochemistry, sterics, kinetics, solvent effects, and so very much more. There are ritzy versions of the SN2 (e.g., Mitsunobu) for those of us that want a little extra sophistication. The SN2 can even cure – or cause – cancer (DNA alkylation).
An Overview of SN2
For anyone stumbling across this that hasn’t brushed up on organic chemistry in a while, an SN2 reaction is a bimolecular nucleophilic substitution reaction. (The “S” and the “N” come from two of those words – I’ll let you guess which ones – and the “2” represents bimolecular). In general, a substitution reaction is one in which a little piece of a molecule falls off and gets replaced by another new piece. The piece that falls off is called a “leaving group” (shown in red below), and the new piece that will get added on is given the less self-explanatory name “nucleophile” (shown in blue below). This reaction is bimolecular because it involves two molecules coming together at the same time – Molecule A and Molecule B.
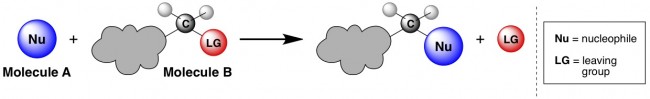
I would argue that an entire organic chemistry course could be taught based on the SN2 reaction**, but here I’ll try to just touch on some of the important principles. Hopefully I can make my case that the SN2 is basically awesome.***
Fundamental Principle #1 – Nucleophilicity/Electrophilicity
 A nucleophile – the blue thing in the scheme above – is something that “loves nuclei” or “loves things that have a positive charge” (the nucleus of an atom is positively charged because that’s where the protons hang out). Unsurprisingly, really good nucleophiles are often themselves negatively charged, because nothing loves a positive like a negative. Conversely, the object of the nucleophile’s attraction (in this case a carbon attached to a leaving group) is called an electrophile – it loves electrons (or things with negative or partial negative charges). This carbon has a partially positive charge… but I won’t get into that here.
A nucleophile – the blue thing in the scheme above – is something that “loves nuclei” or “loves things that have a positive charge” (the nucleus of an atom is positively charged because that’s where the protons hang out). Unsurprisingly, really good nucleophiles are often themselves negatively charged, because nothing loves a positive like a negative. Conversely, the object of the nucleophile’s attraction (in this case a carbon attached to a leaving group) is called an electrophile – it loves electrons (or things with negative or partial negative charges). This carbon has a partially positive charge… but I won’t get into that here.
Fundamental Principle #2 – Stereochemistry
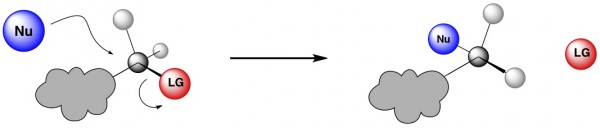
In an SN2, the nucleophile attacks a tetrahedral (sp3 hybridized) carbon atom that is bound to a leaving group (red). Simultaneously, that leaving group takes off. Because the leaving group is still in the process of leaving while the nucleophile is coming in, they can’t both be in the same place at the same time. In fact, the nucleophile attacks the tetrahedral carbon from the exact opposite side of where the leaving group is located. As a result, the stereochemistry of the tetrahedral carbon in the product is inverted from the original. (What the heck is stereochemistry? See here.)
Fundamental Principle #3 – Sterics
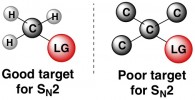
Some carbons that are attached to splendid leaving groups might never be attacked by a nucleophile for an SN2 reaction. The likely cause of this lack of reactivity is sterics, or the presence of nearby molecular clutter that gets in the way of a nucleophile weasling its way in to attack the carbon. At the extremes, a carbon that has three other carbon atoms attached to it is too hindered for SN2 (below right). In an opposite scenario, a carbon with three hydrogens attached (below left) is a prime target for a nucleophile, since those tiny hydrogens don’t get in the way at all.
Fundamental Principle #4 – Kinetics
Because two different molecules must come together at the same time for an SN2 to occur, the rate of the reaction depends on the presence of both molecules. The reaction should go quite fast if there is a lot of both molecules A and B around, because they’re pretty likely to run into each other. If there is a lot of one molecule but not the other, the reaction will be slower. And the reaction will be slowest if there isn’t much of either molecule around. If the rate of a substitution reaction only seems to be affected by the starting concentration of one molecule but not the other, then you know you’re dealing with something other than an SN2.
A Fancy Version – Mitsunobu
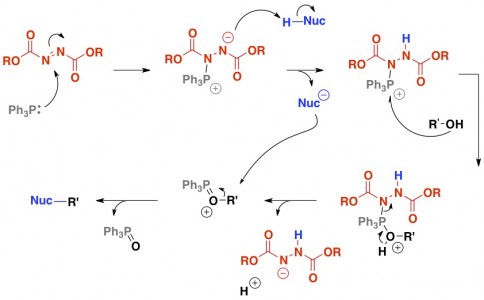
Yup, fancy.
The First Chemotherapy Ever – SN2 in Action
 The prototype chemical treatment for cancer was a nitrogen mustard (HN2). Like most chemotherapy agents, it works by killing cells. That sounds like a bad thing, but it turns out to be a good thing if the victims are cancerous cells. HN2’s cytotoxicity is due to its ability to alkylate DNA (attach a carbon atom/carbon chain onto a place where it doesn’t belong on a DNA strand). It alkylates DNA by a sequence of SN2 reactions, resulting in cross-linkages between portions of DNA.
The prototype chemical treatment for cancer was a nitrogen mustard (HN2). Like most chemotherapy agents, it works by killing cells. That sounds like a bad thing, but it turns out to be a good thing if the victims are cancerous cells. HN2’s cytotoxicity is due to its ability to alkylate DNA (attach a carbon atom/carbon chain onto a place where it doesn’t belong on a DNA strand). It alkylates DNA by a sequence of SN2 reactions, resulting in cross-linkages between portions of DNA.
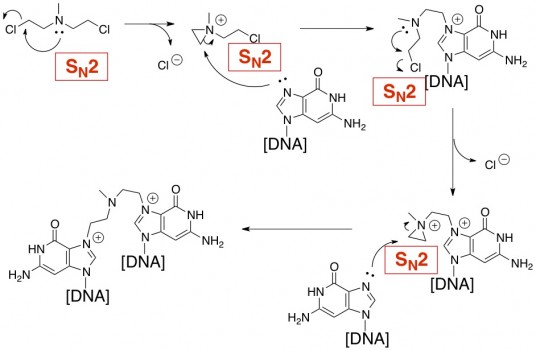
That’s SN2 in a nutshell. Thanks Rachel for the writing prompt!


un related, In our lab we think that your boss´ unique combination of cute and chemical knowledge is bordering on sexy. Do you know if she is available and at which conferences she will be present in the near future. For real. We´ve been discussing it for some time now.