As a new year begins, so do new diet plans. Many diets encourage replacing sugar with artificial sweeteners as a way to cut calories. So what’s really in those pink, blue, and yellow sugar-substitute packets?
Well, most of the content of the artificial sweetener packets is filler material. The actual sweetener molecules are so sweet that you need only a tiny amount, so producers add some extra material (dextrose or maltodextrin) to bring the volume of the packets closer to what you’re used to with sugar.
The sweetener molecules themselves all look pretty different. But to begin with, here’s the structure of real sugar (aka sucrose):
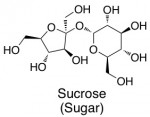
Now let’s compare the structures of the major artificial sweeteners.
Sucralose (Splenda)
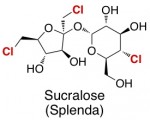 In sucralose’s structure, you can see why Splenda says its “made from sugar, so it tastes like sugar.” The structure is nearly identical to sucrose, except that three “OH” groups have been replaced with chlorines. This change may look small, but it has a dramatic effect on the molecule’s properties.
In sucralose’s structure, you can see why Splenda says its “made from sugar, so it tastes like sugar.” The structure is nearly identical to sucrose, except that three “OH” groups have been replaced with chlorines. This change may look small, but it has a dramatic effect on the molecule’s properties.
This substitution does a couple of things. It makes the “sugar” taste much sweeter, so you need to eat significantly less of it. Secondly, because alkyl chlorides (molecules containing carbon–chlorine bonds are quite rare in nature), the molecule confuses your body’s enzymes, and they can’t figure out how to break it down for energy (or calories).
Aspartame (Nutrasweet, Equal)
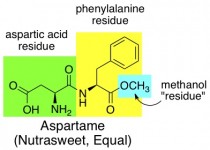 The structure of this molecule looks fairly innocent (which doesn’t necessarily mean anything). It comprises two naturally occuring amino acids, joined together and then capped off with a methyl group. Amino acids are the building blocks of proteins, so your body is pretty familiar with metabolizing structures like this. Your body actually can convert aspartame into energy, so this sweetener is not technically zero-calorie. However, a serving size of aspartame is so small that it contains negligible calories, since aspartame is so very sweet.
The structure of this molecule looks fairly innocent (which doesn’t necessarily mean anything). It comprises two naturally occuring amino acids, joined together and then capped off with a methyl group. Amino acids are the building blocks of proteins, so your body is pretty familiar with metabolizing structures like this. Your body actually can convert aspartame into energy, so this sweetener is not technically zero-calorie. However, a serving size of aspartame is so small that it contains negligible calories, since aspartame is so very sweet.
Saccharin (Sweet’N Low)
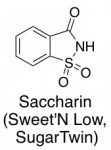 To me, this one is kind of an odd ball. It doesn’t really look like a sugar or a protein, or really any common biomolecule. It looks more like a small molecule that one would find in a chemistry lab (maybe for a Pd-catalyzed diamination).
To me, this one is kind of an odd ball. It doesn’t really look like a sugar or a protein, or really any common biomolecule. It looks more like a small molecule that one would find in a chemistry lab (maybe for a Pd-catalyzed diamination).
Safety concerns about all of these artificial sweeteners have been brought up, so it is worth some investigation before you start consuming them in large quantities.


Good post!
There are legitimate and not-so-legitimate concerns regarding artificial sweeteners. Most of the concerns about sucralose and saccharin have to do with the fact that they’re “not natural”, but for the most part (as far as I can tell) they only have negative side effects at extremely high doses, not the doses you would find in your packets at the coffee shop (as you pointed out already).
Aspartame, on the other hand, does provide some health concerns for those who have phenylketonuria (PKU – http://en.wikipedia.org/wiki/Phenylketonuria) as they cannot break down phenylalanine, which can potentially lead to toxic levels in the body. That’s why there are warnings about diet pop and other things containing phenylalanine!
Thanks for reading, and I agree about sweetener health concerns. If I remember correctly, though I think I read this several years ago, aspartame is one of the most complained-about substance to the FDA, in terms of claimed negative health effects (not just for phenylketonurics).
I think I also read that rats that ingest sucralose don’t excrete all the chlorine you would expect – some chlorines from the sucralose seem to be getting lost in the rat body. Take that as you will…
If one types the single word, “aspartame,” into Google Search with Google’s “Instant” feature turned on, the first three suggestions instantly offered by Google are aspartame side effects, aspartame poisoning and aspartame dangers. Methinks there is controversy lurking in the history of this sweetener … and it is already spread wide all over the internet.
Sharon, what is it about the chlorine that confuses your enzymes? Are there some people whose enzymes seemingly know what how to break it down and thus let it act like a high intensity sugar?
I’m a type 1 diabetic and have been for 24 years. The introduction of sucralose turned out to be almost deadly for me. I tested it on myself after the first incident and here were my findings. When I consume 12 0z of regular (sugar) Coca-Cola in 30 mins starting with a blood sugar of 100 mg/dL, an hour later my blood sugar is around 250 mg/dL. When I consume 12 oz of Diet Coke with Splenda (sucralose) in 30 mins starting with a blood sugar of 100 mg/dL, within 30 mins my blood sugar is around 650 mg/dL. A blood sugar that high is very harmful for the body and the fact that it happens so quickly, within 30 mins of ingestion, is extremely frightening. Do you know any reason this would happen to me, but not to others? For the record, sucralose also affects my aunt in the same way.
Hi Michelle,
I don’t know too much about sucralose metabolism, but I believe that the actual perception of a sweet taste can cause your body to start responding as if you’d been given sugar. I don’t think it means that your enzymes are actually breaking down the sucralose though – I think various hormones or other chemicals are being released into your blood stream because your brain has been tricked into thinking there is sugar around to be processed. Do you know how exactly blood sugar levels are measured? I don’t, but perhaps they don’t actually measure “sugar” per se; they might measure some enzyme or cell marker or something that is typically correlated to blood sugar. In which case, your brain thinks you have sugar, and the blood test is reading the signs that you have sugar, but there’s not actually sugar? If you learn anything more about this I would be interested to hear!
– Sharon