 It’s the holiday season, which means it’s time to start thinking about the flu! The flu is notoriously tricky to prevent with vaccines, partly because there are so many strains of influenza virus, and each strain is constantly undergoing genetic mutations that make it unpredictable. Fortunately, vaccines aren’t the only way to moderate the spread of flu. You’ve heard it all before – wash your hands frequently, don’t stick your fingers in your eyes, cover your face when you sneeze, etc.
It’s the holiday season, which means it’s time to start thinking about the flu! The flu is notoriously tricky to prevent with vaccines, partly because there are so many strains of influenza virus, and each strain is constantly undergoing genetic mutations that make it unpredictable. Fortunately, vaccines aren’t the only way to moderate the spread of flu. You’ve heard it all before – wash your hands frequently, don’t stick your fingers in your eyes, cover your face when you sneeze, etc.
How about coating door knobs and faucets with a paint that makes virus particles explode and spill their genes out?
Yeah, that option sounds pretty good. An article in PNASProceedings of the National Academy of Sciences this week describes how a special “paint” kills viruses that land on it. Unlike using a disinfectant spray – which has to be frequently reapplied – a door handle coated with this paint could theoretically stay sterile even after being touched by a whole slew of sick people. This coating was described a few years ago; but in the current article, researchers investigate just how it works to kill viruses.
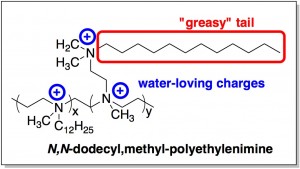
The coating is a polymercalled N,N-dodecyl,methyl-polyethylenimine, shown below. The key feature to note about its structure is the juxtaposition of hydrophobic'scared' of water and hydrophilic'loves' water properties. Molecules that are hydrophobic usually have a structural resemblance to fats or oils. Like fats (which mostly consist of long chains of carbons and hydrogens), hydrophobic molecules don’t want to mix with water. In this polymer, you can see a long “greasy” chain (boxed in red). On the other hand, charged molecules [indicated by a plus and/or minus sign(s)] tend to be attracted to water – think salt (Na+Cl-) dissolving in water. This polymer has charges on it (circled in blue).
Clearly the polymer is a bit conflicted – it’s mostly hydrophobic, but secretly likes water a little bit. Molecules that are confused like this (e.g., detergents) tend to be good at destroying microbes.
The researchers took thin plates of glass or plastic (polypropylene or polyethylene) and coated them with the polymer. They then prepared a solution of influenza virus particles in water, and put a drop of this solution onto the polymer-coated plates. After a few minutes, they rinsed off the plates and analyzed the rinsing to see if the virus particles had survived.
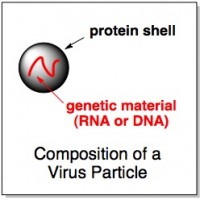 Normally, a virus consists of a little bit of genetic material (usually RNA), encapsulated by a shell of virus proteins. But the researchers found that the rinsings from the plates didn’t contain any virus proteins – just some naked virus genes. This led them to think that the virus particles had all stuck to the polymer-coated plates and then somehow ruptured, leaking their genes back into the water but leaving their proteins attached to the plates.
Normally, a virus consists of a little bit of genetic material (usually RNA), encapsulated by a shell of virus proteins. But the researchers found that the rinsings from the plates didn’t contain any virus proteins – just some naked virus genes. This led them to think that the virus particles had all stuck to the polymer-coated plates and then somehow ruptured, leaking their genes back into the water but leaving their proteins attached to the plates.
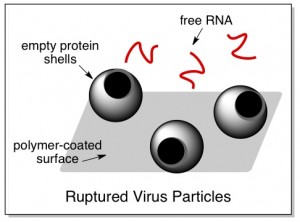
And in fact, a close look at the virus water-treated plates confirmed this suspicion. Using scanning electron microscopy to look at the plates, the researchers saw virus protein shells attached – with giant gaping holes blasted through them. To avoid copyright violations, I won’t show the actual picture here, but the picture below is a cartoon version.
Thus it seems that the antiviral paint works by sticking to viruses and somehow punching holes in them, through which genetic material falls out. This is effective at stopping the spread of the flu, because both naked virus genes and empty virus protein shells are harmless on their own.
It would be interesting to know just how much virus the painted surface can slay before it’s completely coated with virus proteins and no longer effective. Also, it’s not clear to me whether this paint is commercialized yet – I can’t find much about it by Googling, which tentatively indicates it’s not yet commercial (?). Regardless, you should still wash your hands.
Citation:
Bryan B. Hsu, Sze Yinn Wong, Paula T. Hammond, Jianzhu Chen, and Alexander M. Klibanov (2010). Mechanism of inactivation of influenza viruses by immobilized hydrophobic polycations Proceedings of the National Academy of Sciences : 10.1073/pnas.1017012108


Pingback: ResearchBlogging.org News » Blog Archive » Editor’s Selections: Levels of evolution, vaccines are a pain, and killing viruses on contact