 More drama on the Brazilian Blowout front. (For a recap from last time, see here.) For whatever reason, a BB spokesperson emailed me again this weekend with another press release, this one dated December 3rd. @Brazilian Blowout: I’m not sure how you’re finding my blog, but don’t worry, it’s mostly my family reading it and they all have naturally straight hair too. Also, at the end of this post, I will explain why you (BB) should really just embrace the fact that your product contains formaldehyde.
More drama on the Brazilian Blowout front. (For a recap from last time, see here.) For whatever reason, a BB spokesperson emailed me again this weekend with another press release, this one dated December 3rd. @Brazilian Blowout: I’m not sure how you’re finding my blog, but don’t worry, it’s mostly my family reading it and they all have naturally straight hair too. Also, at the end of this post, I will explain why you (BB) should really just embrace the fact that your product contains formaldehyde.
According to this latest press release, BB has gotten an “internationally-recognized scientist” to go to bat for them saying that formaldehyde and methylene glycol should not be counted the same. (They tried to make this argument to OSHA before, apparently they’re persistent.) Doug Schoon of the cosmetics consulting firm Schoon Scientific is taking the side of Brazilian Blowout. [edit: According to Schoon, he is not actually representing BB or any other company] Schoon wrote a detailed letter to Oregon OSHA explaining why he thinks they are wrong. (This link downloads his letter as a pdf from the Schoon website.) Here’s a quote:
The fact that these two unique and different substances exist in chemical equilibrium, does not alter the scientific fact that they are two different substances, with different chemical structures, existing in different chemical families and having different physical properties….Oregon OSHA should accept the chemical reality that Methylene Glycol and Formaldehyde are completely different substances and regulate them accordingly, especially given that Methylene Glycol is a cosmetic ingredient and Formaldehyde is NOT. The public, scientific researchers, and health care professionals would also appreciate receiving accurate and factual information, not dramatically inflated numbers such as those produced by Oregon OSHA.
OK, Doug Schoon, I didn’t want to have to break out Le Châtelier’s principle, but you started it by saying “chemical equilibrium”.
Let’s start with the concept of equilibrium, using a hypothetical reaction in which yellow molecules convert to green molecules. This reaction is reversible, which means that green molecules can also turn back into yellow molecules.
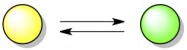
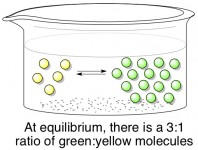
Assuming that both the forward and backward reaction can happen easily, this reaction will quickly reach a state of equilibrium. When a reaction is “in equilibrium”, it means that there is a certain ratio of products (green) to reactants (yellow) that will always be maintained. A green molecule may randomly turn into a yellow molecule from time to time, but just as quickly a yellow molecule will turn into a green one to make up for it. Below, the stable ratio is 3 green molecules for every one yellow molecule. In reality, the ideal ratio for every reaction is different; it depends on the relative energies (stability) of the products and reactants.
Enter Le Châtelier.
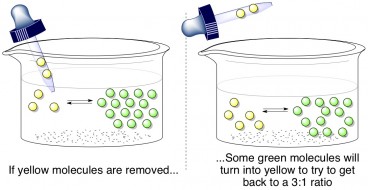
If someone were to yank a few yellow molecules out of the mixture, what would happen? Well, the ideal green:yellow = 3:1 ratio must be maintained, so some of the green molecules will convert into yellow molecules to try to balance the reaction again. This is called Le Châtelier’s principle, which states that a reaction in equilibrium will compensate for any disruptions to its balance. How does this relate to formaldehyde and methylene glycol?
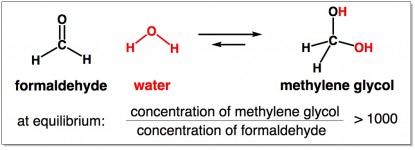
The reaction of formaldehyde with water to make methylene glycol is a reaction that easily reaches equilibrium. The stable ratio favors methylene glycol (more than 1000 methylene glycols for every 1 formaldehyde).
Yet, the “formaldehyde” used to preserve laboratory specimens (such as dead animals) in pickle jars is really a solution of formaldehyde in water, so it could technically be called methylene glycol. When a specimen is dropped into a formaldehyde/methylene glycol solution, the tiny bit of formaldehyde that is present reacts with the biomolecules (proteins, DNA) in the sample. Once those molecules of formaldehyde have reacted, the equilibrium between formaldehyde and methylene glycol is disrupted – there are now too few molecules of formaldehyde left. So, some methylene glycol molecules convert into formaldehyde to balance it all out. These newly formed formaldehyde molecules react with more biomolecules in the sample, and the equilibrium is again disrupted. And so on and so forth until all the methylene glycol has converted into formaldehyde and has reacted with the sample’s biomolecules.
The same could be expected to happen if you soaked your scalp in formaldehyde methylene glycol-containing Brazilian Blowout solution.
 Furthermore, we should remember that there is a another reactant that could be removed, disrupting the equilibrium. Since water is also on the left side of this reaction, removal of water would cause the reaction to go backward to make more formaldehyde (and water). This would happen, for example, by evaporating off the water as steam by heating with a hair dryer.
Furthermore, we should remember that there is a another reactant that could be removed, disrupting the equilibrium. Since water is also on the left side of this reaction, removal of water would cause the reaction to go backward to make more formaldehyde (and water). This would happen, for example, by evaporating off the water as steam by heating with a hair dryer.
So you can thank Le Châtelier for why methylene glycol is considered to be the same thing as formaldehyde, for all intents and purposes.
Lastly, @Brazilian Blowout: don’t be too upset that your product contains formaldehyde. Here’s how to deal with it: Have the hairstylists and the customers wear respirators during application, and maybe stick the head inside a fume hood. Keep the product off the scalp – no need to pickle the client’s skin. The formaldehyde is crucial for making the hair beautiful – it is a preservative after all, and since hair is “dead”, there seems to be no harm in embalming it.
I’m not sure about slow release of formaldehyde from the hair in the days/weeks following application; maybe someone else knows if that should be a concern. Even if Brazilian Blowout is bad for you, people do lots of things that they know are bad for them, like smoking. You’ll still have clients.


If the hair salons which use Brazilian Blowout want to be “green,” is there a way that they could easily recover the formaldehyde from their exhaust ventilation system, so that it could be sold to mortuaries and thereby be re-used in the spirit of recycling to preserve the earth?
Ha. Funny idea, though really there’s not enough formaldehyde given off to capture like that, and the energy input to perform the capture would un-greenify it.
Hi Sharon,
I hope you will allow me to make a correction. I am not representing any company that sells these products. My press releases made that very clear. I am helping some companies, as well as government regulators and activists understand the chemical issues, e.g. to correct MSDS sheets, proper labels, correct testing, recommending proper ventilation, etc. That is my area of expertise, as well as, being a salon safety advocate for the past 20+ years. I can’t help who quotes me, that’s the risks of going public and I don’t mind when they do, as long as I am accurately quoted. Actually, I have been working with many other scientists to help clear up the misconceptions about formaldehyde for more than 7 years, so this is not a new issue.
I fully agree with you that an equilibrium exists and said so repeatedly. I love your graphics, but the equilibrium ratio is not 3 to 1, the ratio is 996 to 4 in favor of Methylene Glycol. Misunderstands like this is what lead Oregon OSHA to originally misreport the levels of free Formaldehyde. That is my only compliant with them. I have a huge respect for OSHA and the important work they do.
Now Oregon OSHA has finally done some air monitoring studies, which is a step in the right direction and the FDA has asked the CIR to looking into both Formaldehyde and Methylene Glycol, so hopefully we can get down to science and move away from speculation based on misinformation. Again, my only interest is to help clear up the long stand misconception that Formaldehyde is a cosmetic ingredient. It is not and never has been! Shampoos, conditioners, body lotions, even nail polish… none of these have ever contained Formaldehyde as an ingredient. That would be impossible, since Formaldehyde is an anhydrous and highly reactive gas. Please feel free to contact me and I’d be happy to discuss this in more detail.
Thanks,
Doug Schoon
Hi Doug, I’ve replied to this comment in this post:
http://icanhasscience.com/chemistry/i-can-has-science-hair-straightener/
Doug, you should probably “help” a misinformed company called Sigma-Aldrich with their MSDS for neutral buffered Formalin solution (Product# HT501128, updated 6/7/11).
They appear to be under the “misconception” that Formaldehyde (CAS# 50-00-0) is an ingredient in their Formalin solution.
You should explain to them how 4/1000 = 0
Pingback: Doug Schoon Responds to Chemical Equilibrium Questions » Clean Air Made Simple
Came across this today: http://ecocentric.blogs.time.com/2011/04/13/warning-getting-your-hair-straightened-could-endanger-your-health/